OPEN-ACCESS PEER-REVIEWED
REVIEW
Angela Tartaglia, Francesca D’Ambrosio, Piera Ramundo, Vincenzo Ferrone, Daniele Ricci,Marcello Locatelli*
University “G. d’ Annunzio” of Chieti-Pescara, Department of Pharmacy, Chieti, Via dei Vestini 31, 66013 Chieti, Italy.
Reviews in Separation Sciences. Vol.2. No.1. pages 19-34 (2020).
Published 15 July 2020. https://doi.org/10.17145/rss.20.003 | (ISSN 2589-1677).
*Correspondence:
Locatelli M . University “G. d’ Annunzio” of Chieti-Pescara, Department of Pharmacy, Chieti, Via dei Vestini 31, 66013 Chieti, Italy.
Editor: Dr. Mervat Hosny, Zagazig University, Zagazig, Egypt.
Open-access and Copyright:
©2020 Tartaglia A et al. This article is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Funding/Manuscript writing assistance:
This work was supported by grant MIUR ex 60%, University of Chieti – Pescara “G. d’Annunzio”, Chieti, Italy. The authors declare that no writing assistance was utilized in the production of this article.
Competing interest:
The authors have declared that no competing interest exist.
Article history:
Received 06 March 2020, Revised 22 May 2020, Accepted 26 May 2020.
Abstract
The QuEChERS method, acronym of quick, easy, cheap, effective, rugged and safe is one of the pre-treatment techniques that has gained great popularity among researchers for analysis of different analytes such as drugs, pesticides, mycotoxin, etc. The QuEChERS method involved different steps: liquid-liquid extraction (LLE) with minimum use of organic solvent, followed by salting-out and clean-up step using dispersive-Solid Phase Extraction (d-SPE). In the last years, all these steps have been optimized in order to increase sensibility and selectivity. This method allows to reduce the sample manipulation, avoiding loss of target analytes and increasing the recovery. Furthermore, the method is considered to be more environmentally friendly, respecting the green analytical chemistry (GAC) principles. The QuEChERS method has already been applied for extraction of different compounds and it is expected that will be more used in the next years. In this review the main advantages and recent applications of this procedure have been reported.
Keywords
QuEChERS, Analytical methods, Chromatographic procedures, Sample preparation, Complex matrices, Green Chemistry.
1.0 Introduction
Nowadays, a challenge that analytical chemists are called to solve is to develop methods that should be easy to perform, faster, more environmentally friendly, precise, accurate, without complicated cleaning steps and that allow the analysis of a wide range of analytes. To resolve this challenge, the Quick, Easy, Cheap, Effective, Rugged and Safe (QuEChERS) method has been developed as one of the most promising friendly, multiclass and multiresidue analytical approach [1-4]. The QuEChERS method was presented for the first time at the European Pesticide Residue Workshop (EPRW) in Rome in 2002 by Anastassiades, Lehotay, Stajnbaher and Schenck and then published in 2003 [1]. The approach proposed by Anastassiades et al. was developed to extract pesticide residues from fruit and vegetables [5,6].
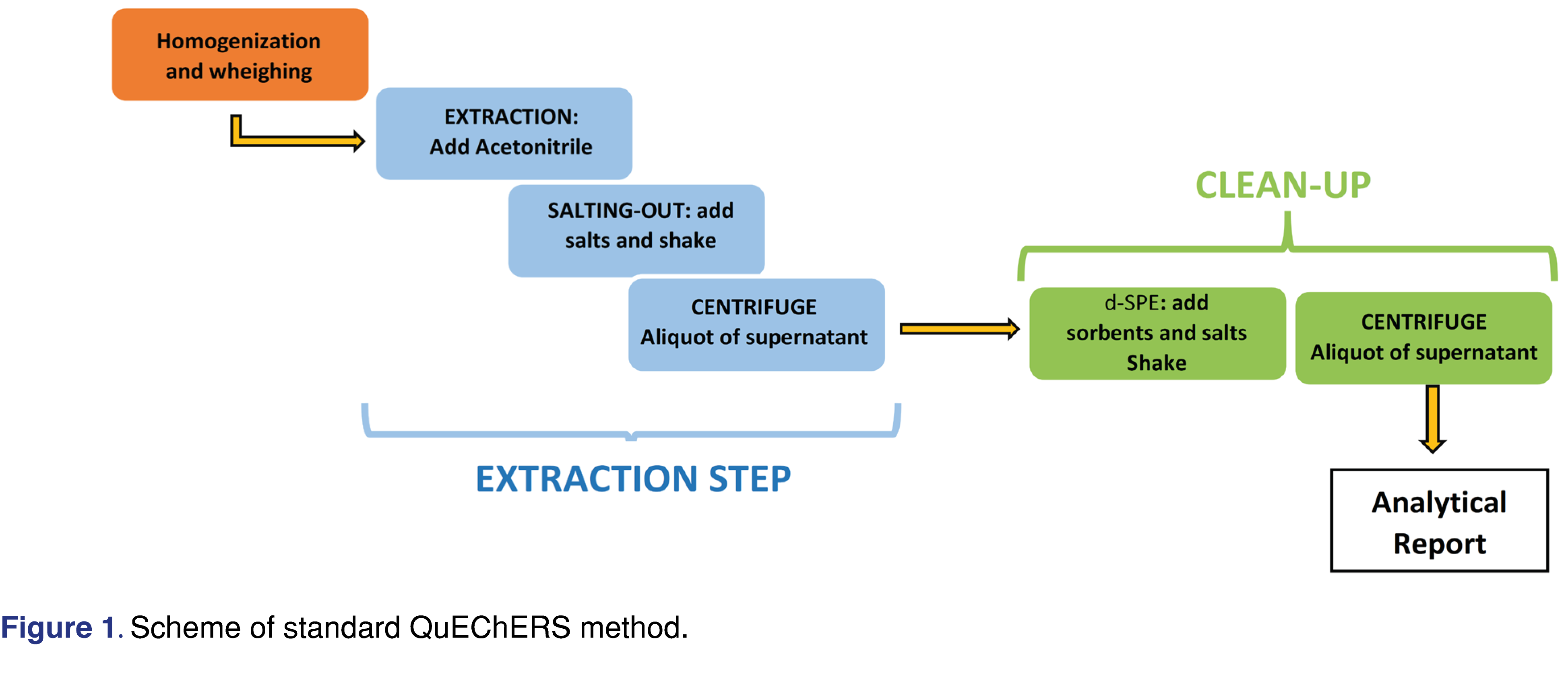
This method rapidly gained the attention of the scientific community due to the ability of extract a broad analyte spectrum, ranging from non-polar to very polar pesticides. Based on its great versatility, recently the QuEChERS approach has become popular over the scientific community, expanding its field of application outside its classical domain into different analytes (e.g. environmental pollutants, amines, polyphenols pharmaceuticals) from different matrices including food, biological fluids and environmental samples [7,8]. In short, the QuEChERS method involved two steps: in the first step acetonitrile (ACN) is added to a solid matrix and a partitioning between the aqueous and organic layer is obtained after adding salts (usually sodium chloride or magnesium chloride); in the second step a combination of salts and porous sorbents is added to the ACN solution obtained before in order to remove matrix interfering substances by dispersive solid phase extraction (d-SPE) [9]. A scheme of standard QuEChERS method is reported in Figure 1.
Before the extraction phase it is necessary to homogenize the sample; it is important to pay attention to the degree of shredding and the quantity of sample as these factors influence the contact surface between the sample and the extracting solvent. Then proceed with the extraction by adding a solvent suitable for the homogenized sample. Several water-miscible solvents were tested for the salting out xtraction/partitioning such as: acetone, acetonitrile, ethanol, ethyl acetate. Different degrees of phase separation were obtained using different concentrations of different salts [10]. Among these solvents, acetonitrile was chosen as best solvent for the first step of QuEChERS method, due to its better phase separation after the addition of salts and its selectivity. Acetonitrile also ensures a low solubility of lipids, thus lipid co-extraction with this technique is relatively limited, but extraction of pesticides from lipids may occurs [11].
In this review paper, QuEChERS methods in several matrices, including food, biological and environmental matrices is reviewed. Whenever possible, the advantages and disadvantages of each extraction method are critically reviewed. Potential readers can gain practical information about QuEChERS method and its possible modification in order to optimize procedures as a function of their research.
2.0 QuEChERS method and its evolution
Since its introduction by Anastassiades et al., in 2003 [1], other two QuEChERS procedures have been developed:
Standard QuEChERS method:
in this method the extraction process involves the addition of 10 mL of ACN to 10 g of powdered sample and shake vigorously. The mixture of salts (MgSO4 and NaCl in a 4:1 ratio) is added to obtain the LLE, it is stirred again for 1 minute and centrifuged at 6000 rpm for 5 minutes. The supernatant is transferred to an Eppendorf containing 150 mg of MgSO4 and 50 mg of PSA (clean-up), shaken for 1 min and centrifuged for 1 min at 6000 rpm. The supernatant is used for analyte research.
AOAC method (the acetate-buffering version):
15 mL of 1% acetic acid in acetonitrile are added to 15 g of homogenized and hydrated sample [12]. The mixture is stirred and 6 g of MgSO4 and 1.5 g of Sodium acetate (strong buffering capacity) were added. It is stirred again and centrifuged for 1 min at 1500 rpm. One mL of supernatant is transferred into Eppendorf containing MgSO4 and PSA (C18 and GCB) for the clean-up phase [13], shake for 30 seconds and spin for 1 min at 1500 rpm. The purified sample is conserved in toluene for GC/MS and in formic acid 6.7 mM for LC-MS/MS.
CEN method (citrate buffer version):
15 mL of 1% acetic acid in ACN were added to 15 g of sample. MgSO4 and NaCl were added in a 4:1 ratio [14] and also citrate buffer (low buffering capacity) to create a pH suitable for inducing LLE. It is stirred for 1 min and is centrifuged for 5 min at 3000 rpm. As sorbent phases of d-SPE are used PSA, C18 and anhydrous MgSO4. It is stirred and centrifuge; the supernatant was preserved in 5% formic acid in ACN and analyzed with GC/MS or LC-MS/MS.
These methods differ for the extraction salts and solvents used. In fact, a first improvement introduced to the QuEChERS method was the optimization of salts amount and their combination based on chemical properties of investigated analytes. Furthermore, CEN and AOAC methods differ from Standard QuEChERS in the use of buffered salts which enhance the recovery of compounds that may be degraded at high or low pH values [9,15]. Salts also play a key role for the liquid-liquid partitioning, due to their interaction with the H2O molecules. The original salts used by Anastassiades were MgSO4 and NaCl although MgCl2, NaNO3, LiCl are options which could be investigated [1]. Magnesium sulphate in contact with the aqueous solution containing the analytes causes an exothermic reaction which leads to an increase in the temperature of the sample and facilitates the extraction of non-polar analytes [16]. Moreover, although magnesium sulphate is a good choice to achieve phase separation, a combination of magnesium sulphate and sodium chloride leads to a better phase separation and it has been also demonstrated that fewer co-extractives were obtained using this combination of salts [17,18]. For this reason, Anastassiades and coworkers developed a non-buffered QuEChERS method using in the first step 10 g of sample, 10 mL of acetonitrile, 4 g of MgSO4 and 1 g of NaCl. The buffered salts have been introduced in the QuEChERS method in order to avoid the degradation of pesticides at high or low pH values. For this reason, the CEN method and the AOAC method has been developed [19-24]. The AOAC method use a combination of 1.5 g of sodium acetate which has a strong buffer capacity and 6 g of MgSO4 in the first step while the CEN methods use 1 g of trisodium citrate dihydrate and 0.5 g of disodium hydrogen citrate sesquihydrate which were added to the standard salt combination (4 g of MgSO4 and 1 g of NaCl). Nowadays, in several laboratories these two methods are widely used as they ensure a sample solution with a pH 5, which is the optimal condition for extracting pH dependent analytes. Moreover, a limitation of AOAC and CEN method may be encountered when samples with a high content of fats are extracted, this is due a higher extraction of interferents which could be less retained by the sorbents in the clan-up step at this pH level.
The second step of QuEChERS method is the clean-up of the extract by using dispersive solid phase extraction (d-SPE). For this purpose, several sorbents have been reported in literature used alone or in combination among primary secondary amine (PSA) octadecyl silica (C18) and graphitized carbon black (GCB) [25]. Historically, the first sorbent used by Anastassiades and coworkers for the cleaning phase was MgSO4 with PSA which ensures effective elimination of fatty acids and organic acids without interfering with the analytes present in the solution. Magnesium sulphate, in this case, was used as drying agent in order to eliminate the water residual in the ACN extract obtained in the first step. Subsequently, both GCB and C18 have been introduced as sorbents. They ensure an efficient removing of fats and pigments such as chlorophyll and carotenoid which could reduce the column life and the efficiency of detectors in the LC or GC systems [26]. Although GCB ensure a high removal of fats, it should be used with caution because GCB remove also analytes with planar structure due to its affinity for planar compounds. Recently, the Enhanced Matrix Removal-Lipid (EMR-Lipid) has been developed by Agilent in order to selectively extract interferents from sample matrix. EMR-Lipid mechanism differs from classical sorbents because it acts through hydrophobic and size exclusion interactions between the sorbent and lipids. Furthermore, unlike classical sorbents which are used after the ACN-Water partitioning, EMR-lipid is used in the first step and subsequently in the second step the EMR-lipid polish was used to achieve the partitioning between ACN and water. This difference is due to the need by EMR-lipid of samples with high water content to achieve an efficient removal of lipids from matrix, in fact a high-water content is impossible to obtain after the ACN-water partitioning [27-29]. Despite the theoretical potential of EMR lipid in the elimination of lipids, other sorbents have been developed that can overcome the lipid removal issue. Another sorbent introduced recently is CarbonX, which remove efficiently more interferents without loss in the analyte recovery compared to GCB sorbent [18]. Finally, thanks to research on graphene-based materials, the Cleanert NANO sorbent based on functionalized carbon nanotubes has been developed. Cleanert NANO allows compared to other sorbent a better removal of fatty acids and pigments. Furthermore, compared to other sorbents, only few milligrams of Cleanert NANO (10-15 mg) are sufficient for the sample clean-up giving to the sorbent the possibility of being packaged as a filter format cartridge.
The most used analytical techniques for the separation and identification of pesticides and organic pollutants are GC and LC coupled with mass spectrometry. In recent years, also ultra-high-performance liquid chromatography (UHPLC) coupled with mass spectrometry has become more popular and widely applied to the analysis of food and environmental samples giving the possibility to the laboratories to increase their workflow. LC is more suitable for the multiresidue analysis of pesticides because it allows the possibility to separate and detect a wide range of compounds while GC is limited to volatile analytes. However, the development of analytical techniques has led to the achievement of rapid analysis due to the tandem MS detection. In fact, the use of GC-MS/MS and LC-MS/MS has increased the use of QuEChERS method giving the possibility to perform the simultaneous analysis of hundreds of compounds [30].
3.0 QuEChERS applications
3.1 Food safety
Food safety and preservation have always been the first goal of the scientific community avoiding contamination and adulteration in order to protect human health. In this field the QuEChERS method represent an alternative tool to conventional analytical methods, due to the possibility of rapid and economic control of different food matrices. The main changes of the standard method mainly concern the extraction solvent, or the mix of salts used. An example is given by Tomas Tuzimski and coworkers [32], a QuEChERS/d-SPE method coupled with HPLC-DAD was optimized for the detection of bisphenols in milk samples both from a can and breast milk. In this work six different sorbent phases and their mixtures (PSA, C18, Z-Sep, Z-Sep Plus, Chitin and EMR LIPID) were evaluated to obtain the best analyte recovery and minimize matrix interferents. In fact, milk samples, due to their generally oily consistency (6% fat content), require special attention during the clean-up phase (d-SPE). Among the various sorbent phases evaluated, Z-Sep (Zirconium dioxide modified silica particles) and Z-Sep Plus were the best because they can adsorb majority of fatty non-polar interferences. Moreover, the introduction of a pre-concentration step before the chromatographic analysis showed an increase in the sensibility of the method. Another example showing the versatility of the QuEChERS technique is reported from Parvin Eslami Shahrbabki et al. [33], where a method has been optimized to determine the acrylamide content in some types of Tha-Dig, a typical meal of Iranian cuisine based on rice, meat and potatoes. Acrylamide, recognized as a human carcinogen by the International Agency for Research on Cancer, can be produced by the reaction of asparagine with sugars at high temperatures; therefore, carbohydrate-rich foods like Tha-Dig are an important source. In this work the authors used a mixture of deionized water, acetonitrile and n-hexane in the extraction phase, while for the clean-up phase the ACN layer was transferred to a falcon containing the sorbent phase (MgSO4 and PSA). Another variant of the QuEChERS method is promoted by Fontana et al. [34]. In their work the analysis of 3-isopropyl-2-methoxypyrazine, 3-sec-butyl-2-methoxypirazine, and 3 isobutyl-2-methoxypirazine in red and white wine, was carried out using toluene as an extraction solvent in the preparation phase of the sample, followed by GC-MS analysis for the determination of different compounds.
As reported in the literature, the QuEChERS method was introduced for the first time for the determination of pesticides in fruit and vegetables. These compounds are widely used in agriculture to eliminate all that damages the cultivated plants and compromises productivity but, if their quantity is higher than the maximum residual level (MRL) imposed by European Union, they could create different health damages; their determination is therefore fundamental in terms of food safety.
Among the various articles on pesticides research in the agri-food sector, the one published by Narenderan et al. [35], reports a modification of the original QuEChERS method; in this work the authors evaluated the presence of five organophosphorus pesticides (OPPs) in some types of fruit and vegetables grown in Nilgiris (South India) by adding to the homogenized sample ACN acidified with 8% of formic acid in order to obtain greater selectivity towards the analytes. A new method for pesticides research but in a different matrix was developed by Stremel et al. [36]. In this work a QuEChERS method was applied for the organochloride pesticides (OCPs) determination in the tissues of various fish trying to optimize the various parameters present in the preparation phase of the sample and using low quantities of sample (0.5 g). The best results in terms of recovery were obtained using acetone and hexane (1:1, v:v) as extracting solvents, MgSO4, NaCl and Na2SO4 in salting-out step and MgSO4, C18 and PSA as sorbent phases for d-SPE. In addition, Chen et al. [37] analyzed plants and soil samples looking for afidopyropen and its metabolites, using a mixture of H2O:ACN (3:10, v:v) for the extraction phase, maintaining MgSO4 and NaCl for the salting-out, but reducing the ratio to 2:1, and using C18, GCB and MgSO4 as sorbent phases.
Always for a tight control of the foods that arrive on our tables, the evaluation of contaminants present in the meat represents a further possibility of application of the QuEChERS method. In fact, it was quickly applied for searching both veterinary drugs and prohibited pharmacologically active substances (substances with hormonal and beta-agonist activities), potentially dangerous for animals and humans health. An example is the work reported by Yen-ping Li et al. [38], in this paper the QuEChERS method was applied applying the acidification of the extracting phase (ACN + 1% of acetic acid) for the determination of -agonists in two different breeding tissues (muscles and viscera). The acidification of the extracting solvent allowed a better recovery of non-polar analytes in the organic phase while the polar impurities of the matrix remained in the aqueous layer. Oliveira et al. (2017) developed a QuEChERS method followed by LC-MS analysis for the detection of various pesticides present in beef. The method was effective for the quantitative determination of more than 150 compounds. The extractive phase was performed with acetonitrile and 1% acetic acid and ethyl acetate (70:30, v:v %), while MgSO4, C18 and PSA were used for the clean-up phase [39].
3.2 Biological samples
Another important application of QuEChERS method is in biological field. This other application includes the determination of antibiotics, veterinary and pharmaceuticals drugs but also pesticides, toxic substances and every kind of contaminant that could be present in biological fluid or human tissues. In literature you can find several works that show the applicability of QuEChERS on both conventional and unconventional matrices, confirming the wide versatility of this preparation method. An example is the study conducted by Admin Wurta et al. [40], to identify methamphetamine from 71 years old man who died of a heart attack [41]. They used centrifuge tubes with PSA, end-capped octadecyl silane and magnesium sulfate for the clean-up step and Captiva nondrip (ND) Lipids cartridge for filtration. The method of standard addition [42] allowed to minimize the matrix effect and exploit advantage of the atomic absorption spectroscopy to the full. Twenty-one solid tissue samples were analyzed like brain, kidney, liver, pancreas, hearth muscle, adipose tissue, as well as different bodily fluids, such as blood. Attention was paid to the location of blood samples analyzed, linking it to post-mortem redistribution of blood. Additionally, it has been seen that, despite the concentration of xenobiotic in the femoral vein, it could reflect that at the time of death (with minimal changes in the post mortem), however it needs taking in consideration the partial loss of xenobiotic through the thin wall of venous vessel, especially in long post mortem range.
In 2016, M. Licata et al. [43], conducted a study on patients suffering acute migraines attacks undergoing therapy, 234 real samples were analyzed with modified QuEChERS method coupled with LC-MS/MS. The study was also widely acknowledged in the toxicological field: as based on the length of the hair, it is possible to make a retrospective assessment about drugs, therapeutic adherence or abuse drugs used up to seven months before, finding a broad consensus in legal and clinical field. In this regard, about fifty psychoactive substances and their metabolites were analyzed, belonging to different pharmacokinetic classes; antidepressants, anxiolytics, mood stabilizers, opioids, and triptans. The authors used methanol instead acetonitrile in the extraction step, then they skipped the partitioning step and only used MgSO4 in the clean-up to extract psychoactive substances. Some particular studies have found variants in the QuEChERS method. Xiao Qian Jia et al. (2019) [44], focused their attention on organic pollutants, especially polycyclic aromatic hydrocarbons (PAHs), organochlorine pesticides (OCPs), and polychlorinated biphenyls (PCBs) which are very harmful for human health, whose presence in serum was searched. During this study different methods of purification were applied to eliminate matrix interferents, (proteins, lipids, carbohydrates, and pigments). A comparison was made between traditional GPC and QuEChERS method, both of them following by a second clean-up step obtained with a combined column of neutral silica gel and neutral alumina oxide (AlO/SiG). The results obtained from the two method are comparable and, only in the case of polychlorinated biphenyls (PCBs) with high molecular weight, the association gel-permeation chromatography and Al2O3/SiG column proved to be convenient after analysis in GC/MS. Instead the work of Lehmann et al. [45] on the exposure of man to pesticides in areas where intensive cultivation of vegetables takes place, is conducted using a non-conventional matrix; the matrix used is human hair that, after a phase of careful homogenization, undergoes extraction using the QuEChERS method and subsequent analysis in GC-MS or UPLC-MS/MS. The optimized procedure involves the use of ACN and H2O (1:1, v:v) in the extractive phases while, for d-SPE, Z-Sep Plus was used as sorbent phase. The study showed high human exposure to locally used pesticides and the optimized protocol was sensitive, accurate and robust.
Another important example of the QuEChERS used on biological samples was carried out by Alves et al., in 2016 [4], for the identification and quantitative determination of fluoxetine, clopramine and their active metabolites in twelve human urine samples of patients being treated with these antidepressants. In this study, the use of ethyl acetate as extraction solvent has showed good results for extraction potential of non-polar compounds, because it ensures the maximum contact efficiency between sample and solvent that allowed satisfying analysis in HPLC. The search for different drugs in the blood through the QuEChERS technique has been exploited by various authors. Pouliopoulos et al. [46] use the traditional method for identifying 15 psychotropic drugs in post-mortem whole blood and serum, while Mizuno et al. [47] use it for testing for valproate only in whole blood. What differs between the two works is the sorbent phase used; in fact the first one used MgSO4 and PSA for d-SPE step, while in the other one MgSO4 and C18 are used.
3.3 Environmental samples
The analysis of soils, sediments, wastewater and surface water represents another large field of application of the QuEChERS technique for the detection of contaminants that could be found in foods of both plant and animal origin. The complexity of the environmental matrix has led to the development of numerous modifications to the standard method.
In this sense Fernandes et al. (2013) [48], in their work modified the QuEChERS version based on the citrate buffer for the determination of 36 pesticides used in organic farming and for the management of pesticides in the soil. These changes mainly concerned the amount of sample used, the adsorbents required and the addition of water at the beginning of the extraction in order to obtain better recoveries. Furthermore, the authors used ultrasound in the extraction step to improve the degree of homogenization of the sample and consequently to have a better extraction.
Another development was that proposed by Wang et al. [49] that in their work for the determination of PAH in the upper, middle and lower layers of soils they used dichloro-methane as the extracting solvent, Na2SO4 as salt, PSA, C18 and Na2SO4 as adsorbents for the purification step. A further application of the QuEChERS acidified method in the environmental field is reported from Kachawaha and coworkers [50] for the analysis of pharmaceutical and personal care products (PPCPs) in surface water and sewage. In this work ACN acidified with formic acid as extraction solvent, MgSO4 and NH4CH3CO2 for the breakdown were used, but the purification phase was not performed.
Figures and Tables
[Click to enlarge]
3.4 Mycotoxins
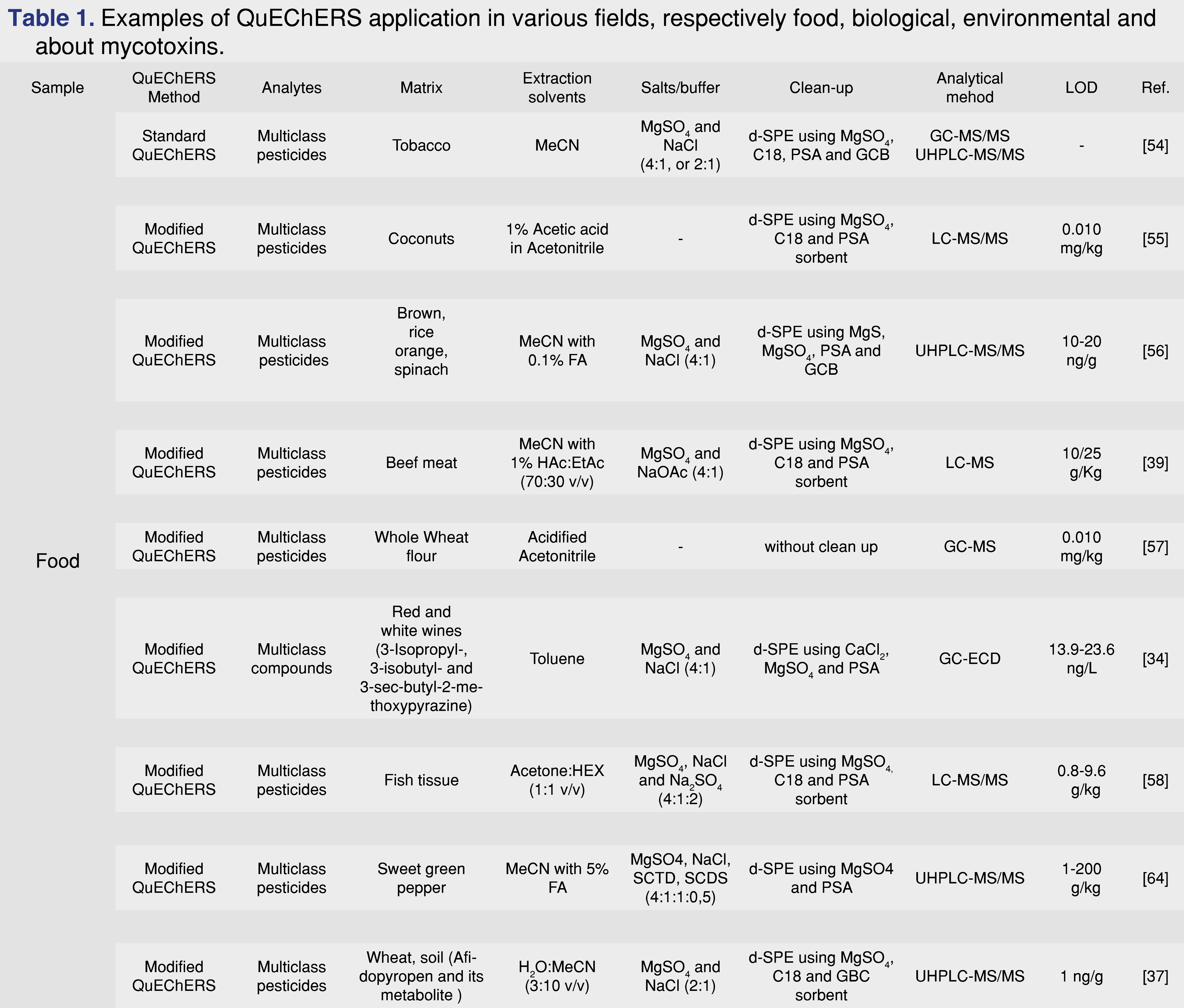
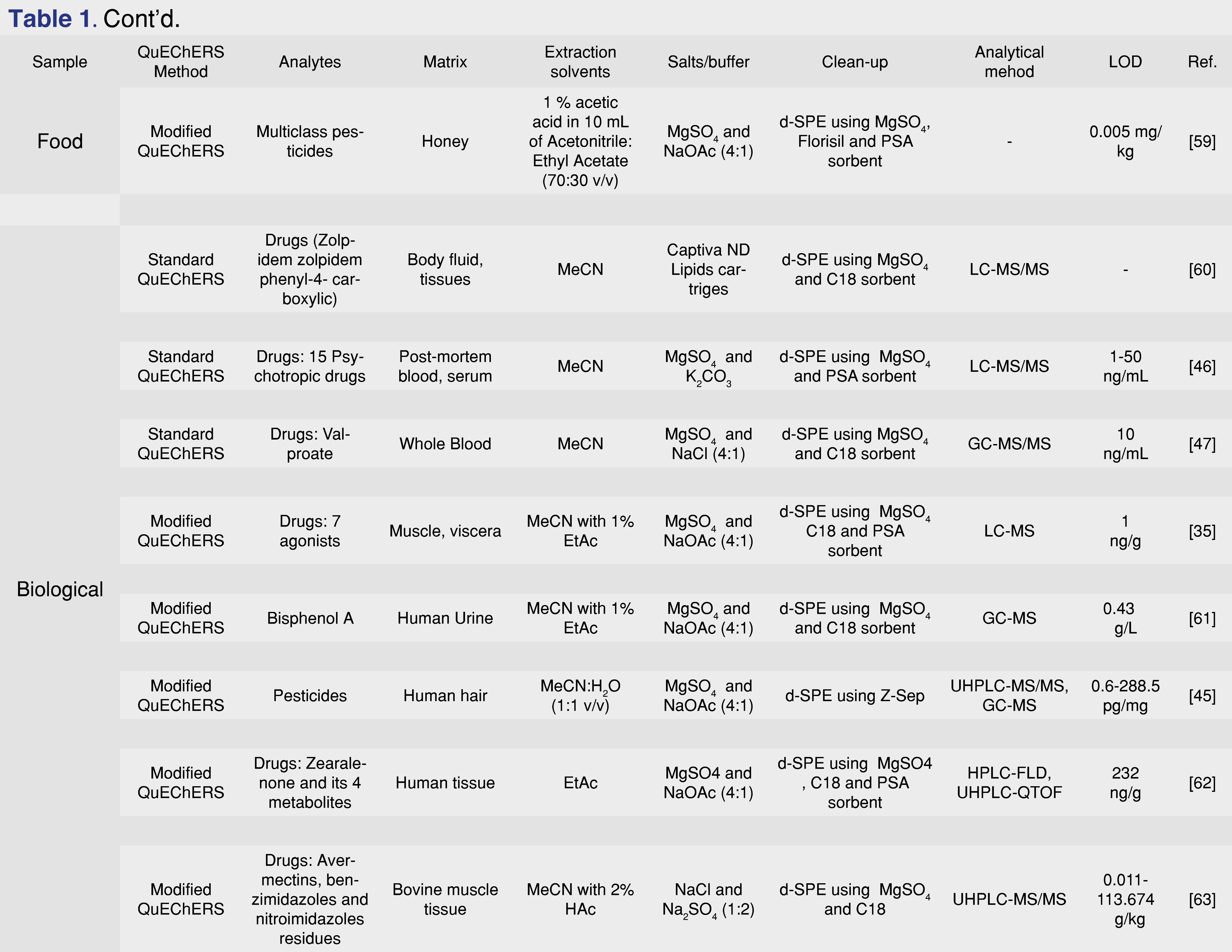
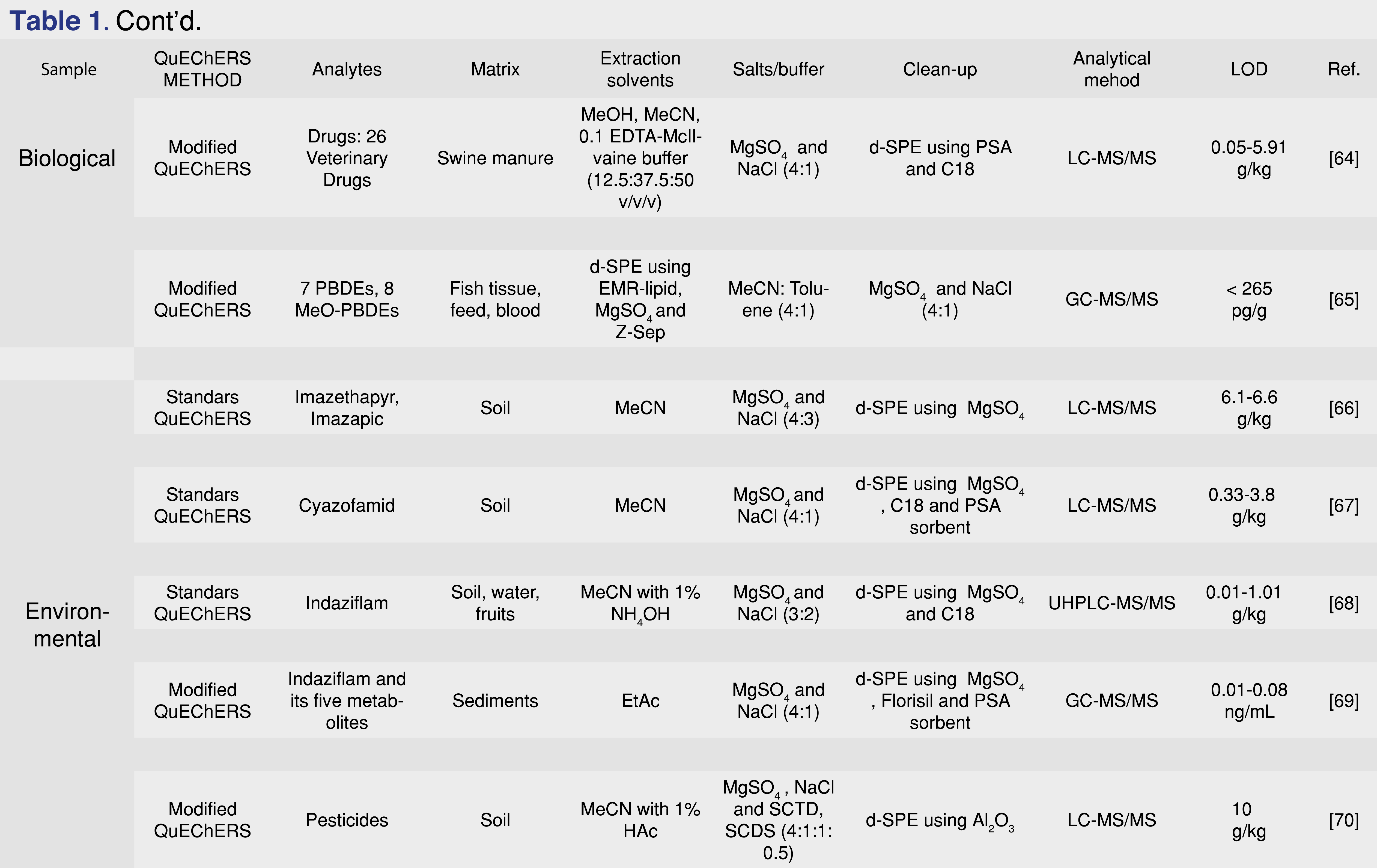
Mycotoxins are chemicals with potential toxicity, produced by fungi, molds and various microscopic species. Environmental and food contamination due to the spread of these substances can generate even chronic toxicity phenomena, putting human and animal health at risk. Therefore, the identification of such substances is indispensable to limit poisoning phenomena. The QuEChERS method fits into this field thanks to its extreme versatility and, although with different modifications compared to the traditional method, is a fast and advantageous technique. In the literature there are several works in which the QuEChERS technique has been used for the determination of mycotoxins. An example is the work of Martins et al. [51] in which the research of twelve mycotoxins and their respective metabolites in breakfast cereals was conducted using ACN as extraction solvent, MgSO4 and NaCl (4:1) in the salting-out phase and MgSO4 and PSA as sorbent phases for d-SPE. Also, in the food field, Huang et al. [52] have researched different mycotoxins present in licorice using a QuEChERS method modified by the addition of formic acid to the acetonitrile and using MgSO4, NaCl, SCTD and SCDS (4:1:0.5:1) as salts. Wang et al. [53], instead they modified the standard QuEChERS method by acidifying acetonitrile with citric acid for the research of various mycotoxins in dried fruit. In addition to the applications abovementioned, QuEChERS method was applied also for different other drugs, mycotoxins, various environmental contaminants, samples of animal origin like beef, pork, sheep meat, but also eggs, honey, aquatic organism (Table 1). Then it could be applied for multi-classes analysis, for the detention of large range of contaminants with a single analysis.
4.0 Comparison with other extraction methods
Due to the wide range of application of QuEChERS method, its comparison with other extraction techniques is allowed. A comparison between QuEChERS, Soxhlet and solid-liquid extraction (SLE) is presented by Durovic-Pejcev et al. for the analysis of multiclass pesticides in soil samples. The traditional techniques are SLE and Soxhlet which are time consuming, tedious, expensive and require large quantities of organic solvents. Among these techniques QuEChERS showed much better results in terms recovery, LOD values and relative standard deviations (RSDs) clearly indicating that SLE and soxhlet extraction still needs improvements for the determination of multiclass pesticides in soil samples [74]. Chen et al, on the contrary, reported that micellar extraction combined with ionic liquid-based vortex-assisted liquid liquid microextraction (ME-IL-VALLME) achieved better analytical performance with higher enrichment factor compared with QuEChERS for the analysis of difeconazole in cowpea [75]. An interesting comparison was made by Pawliszyn et al. between solid-phase microextraction, solvent extraction (SE) and QuEChERS for the quantitative analysis of veterinary drug residues in chicken and beef matrices. In terms of time required to perform extraction SE requires two min/sample, QuEChERS requires 3 min/sample and SPME less than 1 min/sample. Furthermore, SPME, can be easily automated eliminating a significant source of variation. The low consumption of organic solvent is clearly an advantage of solid phase microextraction, in fact the SPME protocol requires only 0.3 mL of organic solvent compared to the QuEChERS method which requires 10 mL of ACN. However, a careful optimization of several parameters such as extraction phase, desorption step, adsorption time is needed when a SPME is performed. Finally, SPME obtained similar performance compared to QuEChERS and SE [76]. Another interesting comparison between, protein precipitation, SPE and QuEChERS was made by Kim et al. for the analysis of nitrosable pesticides in human serum and urine by liquid chromatography-orbital ion trap mass spectrometry [77]. Among these sample preparation techniques, deproteinization by methanol led to an excessive ion enhancement of some analytes causing the suppression of others. Solid-phase extraction was also evaluated, three types of sorbents were evaluated. Although SPE is recognized as a valid extraction technique capable of obtaining much cleaner extracts and high accuracy, in this case it has achieved a significant loss for some analytes. Kin et al. in their work demonstrated that QuEChERS approach is a suitable method for the extraction of nitrosable pesticides in human biofluids. Furthermore, QuEChERS method resulted in higher recovery among the tested extraction techniques showing minimal matrix effects in both the matrices. An exhaustive comparison between soxhlet extraction, microwave assisted extraction, ultrasound assisted extraction and QuEChERS has been reported by Forbes et al. in the analysis of polycyclic aromatic hydrocarbons (PAH) from lichen biomonitors [78].
Among the investigated techniques, QuEChERS and MAE performed better compared to UAE and Soxhlet extraction. Furthermore, it was found that hexane-acetone mixture (1:1, v:v) which is never reported before for the extraction of PAH achieved the highest total peak area for all the PAH of interest and a relative standard deviation equal or better of the other extraction techniques. Although the microwave extraction is uncommon for a matrix as lichens, it has widely performed to extract PAH from several matrices achieving performance comparable with QuEChERS method. Microwave assisted extraction was found to perform better than QuEChERS, Soxhlet extraction and accelerated solvent extraction of steroid estrogens from sediments [79]. In Table 2, a comparison on the efficiency of different extraction approaches with the QuEChERS method is reported.
5.0 Conclusions
The QuEChERS approach is presented as a rapid, economical, and very advantageous sample preparation technique in terms of environmental safety. From the initial limited use of pesticide research in fruit and vegetables, it has been possible to extend its application in various other fields, from food to pharmaceutical, environmental and forensic, thanks above all to its versatility. The ease in preparing the sample thanks to only two steps, extraction and clean up, is the main advantage of the method which guarantees quality, safety, authenticity and traceability of the analytes in the various matrices. Through the control of various factors, such as the extraction solvent, the sample quantity, the sample/solvent ratio, the pH, the salts and the sorbent phases, the QuEChERS protocol guarantees a high recovery rate and a better analytical performance compared to other conventional extraction techniques. Reliability and reproducibility make QuEChERS the procedure of excellence in the pretreatment phase of samples destined for analysis by chromatography or spectrometry. The future perspectives are aimed at reducing the manual steps turning their attention to the automation and miniaturization of the method, to speed up the analysis time, and to increase the efficiency and the selectivity through the coupling to other preconcentration methods.
6.0 References
- Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int 86(2), 412–431 (2003). [CrossRef]
- Sell B, Sniegocki T, Zmudzki J, Posyniak A. Development of an analytical procedure for the determination of multiclass compounds for forensic veterinary toxicology. J Anal Toxicol 42(3), 183–191 (2018). [CrossRef]
- Galuszka A, Migaszewski Z, Namiesnik J. The 12 principles of green analytical chemistry and the significance mnemonic of green analytical practices. Trends Anal Chem 50, 78–84 (2013). [CrossRef]
- Alves V, Conceição C, Gonçalves J, Teixeira HM, Câmara JS. Improved Analytical Approach Based on QuEChERS/UHPLC-PDA for Quantification of Fluoxetine, Clomipramine and their Active Metabolites in Human Urine Samples. J Anal Toxicol 41(1), 45–53 (2017). [CrossRef]
- Lehotay SJ, de Kok A, Hiemstra M, Van Bodegraven P. Validation of a fast and easy method for the determination of residues form 229 pesticides in fruits and vegetables using gas and liquid chromatography and mass spectrometric detection. J AOAC Int 88, 595-614 (2005). [CrossRef]
- Lee J, Kim L, Shin Y, Lee J, Lee J, Kim E, Moon JK, Kim JH. Rapid and simultaneous analysis of 360 pesticides in brown rice, spinach, orange, and potato using microbore GC-MS/MS. J Agric Food Chem 65, 3387-3395 (2017). [CrossRef]
- Gonzalez-Curbelo MA, Socas-Rodriguez B, Herrera-Herrera AV, Gonzalez-Salamo J, Hernandez-Borges, Rodriguez-Delgado MA. Evolution and applications of the QuEChERS method. Trends Anal Chem 71, 169-185 (2015). [CrossRef]
- Grimalt S, Dehouck P. Review of analytical methods for the determination of pesticide residues in grapes. J Chromatogr A 1433, 1-23 (2016). [CrossRef]
- Lehotay SJ. Determination of pesticide residues in foods by acetonitrile extraction and partitioning with magnesium sulfate: collaborative study. J AOAC Int 90, 485-520 (2007). [CrossRef]
- Schenck FJ, Callery P, Gannet PM, Daft JR, Lehotay SJ. Comparison of magnesium sulfate and sodium sulfate for removal of water from pesticide extracts of foods. J AOAC Int 85, 1177-1180 (2002). [CrossRef]
- Lopez-Blanco R, Nortes-Mendez R, Robles-Molina J, Moreno-Gonzalez D, Gilbert-Lopez B, Garcia-Reyes JF, Molina-Diaz A. Evaluation of different cleanup sorbents for multiresidue pesticide analysis in fatty vegetable matrices by liquid chromatography tandem mass spectrometry. J Chromatogr A 1456, 89-104 (2016). [CrossRef]
- Hou Y, Chen H, Li X, Liao Y, Tsunoda M, Zhang Y, Deng S, Song Y. A Modified QuEChERS Method for Determination of Pyrethroid Residues in Traditional Chinese Medicine Oral Liquids by High-Performance-Liquid Chromatography. Molecules 24(8), 1470–1484 (2019). [CrossRef]
- He Z, Wang L, Peng Y, Luo M, Wang W, Liu X. Multi-residue analysis of over 200 pesticides in cereals using a QuEChERS and gas-chromatography-tandem mass spectrometry-based method. Food Chem 169, 372–380 (2015). [CrossRef]
- Lee YJ, Rahman MM, Abd El-Aty AM, Choi JH, Chung HS, Kim SW, Shin HC, Shin JH. Detection of three herbicide, and one metabolite, residues in brown rice and rice straw using various versions of the QuEChERS method and liquid chromatography- tandem mass spectrometry. Food Chem 210, 442–450 (2016). [CrossRef]
- Lehotay SJ, Mastovska K, Lightfield AR. Use of buffering and other means to improve results of problematic pesticides in a fast and easy method for residue analysis of fruits and vegetables. J AOAC Int 88, 615 (2015). [CrossRef]
- Lehotay SJ. QuEChERS Sample Preparation Approach for Mass Spectrometric Analysis of Pesticide Residues in Foods. Methods Mol Biol. 747, 65-91 (2011). [CrossRef]
- Gonzalez-Curbelo MA, Lehotay SJ, Hernandez-Borges J, Rodriguez-Delgado MA. Use of ammonium formate in QuEChERS for high throughput analysis of pesticides in food by fast, low-pressure gas chromatography and liquid chromatography tandem mass spectrometry. J Chromatogr A 1358, 75-84 (2014). [CrossRef]
- Rejczak T, Tuzimski T. A review of recent developments and trends in the QuEChERS sample preparation approach. Open Chem J 13, 980-1010 (2015). [CrossRef]
- Dusek M, Jandovska V, Cermak P, Mikyska A, Olsovska J. A novel approach for identification of biologically active phenolic compounds in complex matrices using hybrid quadrupole-orbitrap mass spectrometer: a promising tool for testing antimicrobial activity of hops. Talanta 156-157, 209-217 (2016). [CrossRef]
- Hamed AM, Moreno-Gonzalez D, Gamiz-Gracia L, Garcia-Campana AM. Evaluation of a new modified QuEChERS method for the monitoring of carbamate residues in high-fat cheeses by using UHPLC-MS/MS. J Sep Sci 40, 488-496 (2017). [CrossRef]
- Cladiere M, Delaporte J, Le Roux E, Camel V. Multi-class analysis for simultanoues determination of pesticides, mycotoxins, process-induced toxicants and packaging contaminants in tea. Food Chem 242, 113-121 (2018). [CrossRef]
- Rahman MM, Lee HS, Abd El-Aty AM, Kabir MH, Chung HS, Park JH, Kim MR, Kim J,Shin HC, Shin SS, Shim JH. Determination of endrin and δ-keto endrin in five food products of animal origin using GC-µECD: A modified QuEChERS approach to traditional detection. Food Chem 263, 59-66 (2018). [CrossRef]
- Chawla S, Patel DJ, Patel SH, Kalasariya RL, Shah PG. Behaviour and risk assessment of fluopyram and its metabolite in cucumber (Cucumis sativus) fruit and in soil. Environ Sci Pollut Control Ser 25, 11626-11634 (2018). [CrossRef]
- Martinez-Piernas AB, Polo-Lopez MI, Fernandez-Ibanez P, Aguera A. Validation and application of a multiresidue method based on liquid chromatography-tandem mass spectrometry for evaluating the plant uptake of 74 microcontaminants in crops irrigated with treated municipal waste-water. J Chromatogr A 1534, 10-21 (2018). [CrossRef]
- Cabrera L, Caldas SS, Prestes OD, Primel EG, Zanella R. Evaluation of alternative sorbents for dispersive solid-phase extraction clean-up in the QuEChERS method for the determination of pesticides residues in rice by liquid chromatography with tandem mass spectrometry. J Sep Sci 39, 1945-1954 (2016). [CrossRef]
- Lankova D, Kockovska M, Lacina O, Kalachova K, Pulkrabova J, Hajslova J. Rapid and simple method for determination of hexabromocyclododecanes and other LC-MS-MS amenable brominated flame retardants in fish. Anal Bioanal Chem 404, 7829-7839 (2013). [CrossRef]
- Han L, Matarrita J, Sapozhnikova Y, Lehotay SJ. Evaluation of a recent product to remove lips and other matrix co-extractives in the analysis of pesticide residues and environmental contaminants in foods. J Chromatogr A 1449, 17-29 (2016). [CrossRef]
- Zhao L, Lucas D. Multiresidue analysis of pesticides in avocado with Agilent bond elut EMR-lipid by GC-MS/MS. Available at https://www.agilent.com/cs/library/applications/5991-6097EN.pdf
- Zhao L, Lucas D. Multiresidue analysis of veterinary drugs in bovine liver by LC-MS/MS Agilent bond elut enhanced matrix removal-lipid authors. Available at https://www.agilent.com/cs/library/applications/5991-6096EN.pdf
- Agela technologies Cleanert® SPE SPE cartridges and plates SPE bulk media clean-up cartridges for Ion chromatography accessories applications. Available at http://agela.com/product/detail/196
- Zhao MA, Feng YN, Zhu YZ, Kim JH. Multi-residue method for determination of 238 pesticedes in Chinese cabbage and cucumber by liquid chromatography-tandem mass spectrometry: Comparison of different purification procedures. J Agric Food Chem 62, 11449 (2014). [CrossRef]
- Tuzimski T, Szubartowski S. Method Development for Selected Bisphenols Analysis in Sweetened Condensed Milk from a Can and Breast Milk Samples by HPLC–DAD and HPLC-QqQ-MS: Comparison of Sorbents (Z-SEP, Z-SEP Plus, PSA, C18, Chitin and EMR-Lipid) for Clean-Up of QuEChERS Extract. Molecules 24(11), 2093–2113 (2019). [CrossRef]
- Shahrbabki PE, Hajimohammadi B, Shoeibi S, Elmi M, Yousefzadeh A, Conti GO, Ferrante M, Amirahmadi M, Fakhri Y, Khaneghah AM. Probabilistic non-carcinogenic and carcinogenic risk assessments (Monte Carlo simulation method) of the measured acrylamide content in Tah-dig using QuEChERS extraction and UHPLC-MS/MS. Food Chem Toxicol. 118, 361–370 (2018). [CrossRef]
- Fontana AR, Bottini R. QuEChERS methods for the determination of 3-alky-2-methoxypyrazines in wines by gas chromatography-mass spectrometry. Food Anal Methods 9(12), 3352–3359 (2016). [CrossRef]
- Narenderana ST, Meyyanathana SN, Venkata Satyanarayana Reddy Karrib V, Babua B, Pavankumar Chintamanenic. Multivariate response surface methodology assisted modified QuEChERS extraction method for the evaluation of organophosphate pesticides in fruits and vegetables cultivated in Nilgiris. South India. Food Chem 300, 1–8 (2019). [CrossRef]
- Stremel TRDO, Domingues CE, Zittel R, Silva CP, Weinert PL, Monteiro FC, Campos SX. Development, validation and matrix effect of a QuEChERS method for the analysis of organochlorine pesticides in fish tissue. J Environ Sci Heal B 53(4), 246–254(2018). [CrossRef]
- Chen Y, Guo M, Liu X, Xu J, Dong F, Wu X, Li B. Zheng. Determination and dissipation of afidopyropen and its metabolite in wheat and soil using QuEChERS-UHPLC-MS/MS. J Sep Sci 41 1674–1681 (2018). [CrossRef]
- Lin YP, Lee YL, Hung CY, Huang WJ, Lin SC. Determination of multiresidue analysis of β-agonists in muscle and viscera using liquid chromatograph/ tandem mass spectrometry with Quick, Easy, Cheap, Effective, Rugged, and Safe methodologies. J Food Drug Anal 25(2), 275–284 (2017). [CrossRef]
- Oliveira FADS, Pereira ENC, Gobbi JM, Soto-Blanco B, Melo MM. Multiresidue method for detection of pesticides in beef meat using liquid chromatography coupled to mass spectrometry detection (LC-MS) after QuEChERS extraction. Food Addit Contam 35(1), 94–109 (2018). [CrossRef]
- Wurita A, Hasegawa K, Minakata K, Gionmori K, Nozawa H, Yamagishi I, Suzuki O, Watanabe K. Post mortem redistribution of methamphetamine and amphetamine in blood specimens from various blood vessels and in the specimens from pericardial fluid, bile, stomach contents and various solid tissues collected from human cadaver. Forensic Toxicol 34(1), 191–198 (2016). [CrossRef]
- Usui K, Hayashizaki Y, Hashiyada M, Funayama M. Rapid drug extraction from human whole blood using a modified QuEChERS extraction method. Leg Med 14(6), 286–296 (2012). [CrossRef]
- Wurita A, Hasegawa K, Minakata K, Gonmori K, Nozawa H, Yamagishi I, Suzuki O, Watanabe, K. Postmortem distribution of a-pyrrolidinobutiophenone in body fluids and solid tissues of a human cadaver. Leg Med 16(5), 241–246 (2014). [CrossRef]
- Licata M, Rustichelli C, Palazzoli F, Ferrari A, Baraldi C, Vandelli D, Verri P, Marchesi F, Silingardi E. Hair testing in clinical setting: Simultaneous determination of 50 psychoactive drugs and metabolites in headache patients by LC tandem MS. J Pharm Biomed Anal 126, 14–25 (2016). [CrossRef]
- Jia X, Yin S, Xu J, Li N, Ren M, Qin Y, Zhou J, Wei Y, Guo Y, Gao M, Yu Y, Wang B, Li Z. An efficient method to simultaneously analyze multi-class organic pollutants in human serum. Environ Pollut 251, 400–406 (2019). [CrossRef]
- Lehmann E, Oltramare C, de Alencastro LF. Development of a modified QuEChERS method for multi-class pesticide analysis in human hair by GC-MS and UPLC-MS/MS. Anal Chim Acta 999, 87–98 (2018). [CrossRef]
- Pouliopoulos A, Tsakelidou E, Krokos A, Gika HG, Theodoridis G, Raikos N. Quantification of 15 psychotropic drugs in serum and post-mortem blood sample after a modified mini-QuEChERS by UHPLC-MS/MS. J Anal Toxicol 42(5), 337–345 (2018). [CrossRef]
- Mizuno S, Lee XP, Fujishiro M, Matsujama T, Yamada M, Sakamoto Y, Kusano M, Zaitsu K, Hasegawa C, Hasegawa I, Kumazawa T, Ishii A, Sato K. High-throughput determination of valproate in human samples by modified QuEChERS extraction and GC-MS/MS. Leg Med 31, 66–73 (2018). [CrossRef]
- Fernandes VC, Domingues VF, Mateus N, Delerue-Matos C. Multiresidues pesticides in soils using modified QuEChERS with disposable pipette extraction and dispersive solid-phase extraction. J Sep Sci 36(2), 376–382 (2013). [CrossRef]
- Wang D, Ma J, Li H, Zhang X. Concentration and potential ecological risk of PAHs in different layers of soil in the petroleum-contaminated areas of the Loess Plateau, China. Int J Environ Res Public Health 15(8), 1785–1800 (2018). [CrossRef]
- Kachhawaha AS, Nagarnaik PM, Jadhav M, Pudale A, Labhasetwar PK, Banerjee K. Optimization of a modified QuEChERS method for multiresidue analysis of pharmaceuticals and personal care products in sewage and surface water by LC-MS/MS. J AOAC Int 100(3), 592–597 (2017). [CrossRef]
- Martins C, Assunçao R, Cunha SC, Ferdandes JO, Jager A, Petta T, Oliveira CA, Alvito P. Assessment of multiplot mycotoxins in breakfast cereals available in the Portoguese market. Food Chem 239, 132–140 (2018). [CrossRef]
- Huang X, Wang S, Mao D, Miao S, Hu Q, Ji S. Optimized QuEChERS method combined with UHPLC-MS/MS for the simultaneous determination of 15 mycotoxins in liquorice. J AOAC Int 101(3), 633–642 (2018). [CrossRef]
- Wang Y, Nie J, Yan Z, Li Z, Cheng Y, Chang W. Occurrence and co-occurrence of mycotoxins in nuts and dried fruits from China. Food Control 88, 181–189 (2018). [CrossRef]
- Bernanrdi G, Kemmerich M, Ribeiro LC, Adaime MB, Zanella R, Prestes OD. An effective method for pesticide residues determination in tobacco by GC-MS/MS and UHPLC-MS/MS employing acetonitrile extraction with low-temperature precipitation and d-SPE clean up. Talanta 161, 40–47 (2016). [CrossRef]
- Ferreira JA, Ferreira JMS, Talamini V, Flacco JF, Rizzetti TM, Prestes OD, Adaime MB, Zanella R, Bottoli CBG. Determination of pesticides in coconut (Coconut nucifera Linn.) water and pulp using modified QuEChERS and LC-MS/MS. Food Chem 213, 616–624 (2016). [CrossRef]
- Lee J, Shin JL, Kim BJ, Kim JH. Simultaneous analysis f 310 pesticide multiresidues using HPLC-MS/MS in brown rice, orange and spinach. Chemosphere 207, 519–526 (2018). [CrossRef]
- Bordin AB, Minetto L, Filho IN, Beal LL. Determination of pesticide residues in whole wheat flour using modified QuEChERS and LC-MS/MS. Food Anal Methods 10(1), 1–9 (2017). [CrossRef]
- De Oliveira Stremel TR, Dominigues CE, Zittel R, Silva CP, Weinert PL, Monteiro FC, Campos SX, Silva P. Pesticides, food, contaminants and agricultural wastes. Development, validation and matrix effect of a QuEChERS method for the analysis of organochlorine pesticides in fish tissue. J Environ Sci and Health Part B 53(4) 246–254 (2017). [CrossRef]
- Tette PAS, de Silva Oliveira FA, Pereira ENC, Silva G, Gloria MBA, Fernandes C. Multiclass method for pesticides quantification in honey by means of modified QuEChERS and UHPLC-MS/MS. Food Chem 211, 130–139 (2016). [CrossRef]
- Hasegawa K, Wurita A, Nozawa H, Yamagishi I, Minakata K, Watanabe K, Suzuki O. Fatal Zolpidem poisoning doing due to intravenous self-injection: postmortem distribution/redistribution of Zolpidem and its predominant metabolite zolpidem phenyl-4-carboxylic acid in body fluid and solid tissues in an autopsy case. Forensic Sci Int 290, 111–120 (2018). [CrossRef]
- Correia-Sà L, Norberto S, Delerue-Matos C, Calhau C, Dominigues VF. Micro-QuEChERS extraction coupled to GC-MS for a fast determination of Bisphenol A in human urine. J Chromatogr B Analyt Technol Biomed Life Sci 1072, 9–16 (2018). [CrossRef]
- Pajewska M, Lojko M, Cendrowski K, Sawicki W, Kowalkowski T, Buszewski B, Gadzala-Kopciuch, R. The determination of zearalenone and its major metabolites in endometrial cancer tissues. Anal Bioanal Chem 410(5), 1571–1582 (2018). [CrossRef]
- Silva GRD, Lima JA, Souza LF, Santos FA, Lana MAG, Assis DCS, Cancado SV. Multiresidue method for identification and quantification of avermectins, benzimidazoles and nitroimidazoles residues in bovine muscles tissue by ultra-high-performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) using a QuEChERS approach. Talanta 171, 307–320 (2017). [CrossRef]
- Guo C, Wang M, Xiao H, Huai B, Wang F, Pan G, Liao X, Liu Y. Development of a modified QuEChERS method for the determination of veterinary antibiotics in swine manure by liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 1027, 110–118 (2016). [CrossRef]
- Cruz R, Marques A, Casal S, Cunha SC. Fast and environmental-friendly methods for the determination of polybrominated diphenyl ethers and their metabolites in fish tissues and feed. Sci Total Environ 646, 1503–1515 (2019). [CrossRef]
- da Costa Marinho MI, Costa AIG, Vieira NM, Paiva MCG, de Freitas FCL, da Silva AA. Validation and Application of a QuEChERS based method for estimation of half-life of imidazolinone herbicide in soils by LC-ESI-MS/MS. Ecotoxicol Environ Saf 167, 212–217 (2019). [CrossRef]
- Xu Z, Zhang C, Yu J, Zhang C, Wu M, He H, Zhu Y, Lou F, Wu Y, Wang Y, Chen L, Zhao H, Wang Q, Cai L. Field investigation of dissipations and residues of cyazofamid in soil and tomato: risk assessment of human exposure to cyazofamid via tomato intake. Environ Sci Pollut Res 24, 3483–3492 (2017). [CrossRef]
- Hu M, Qiu J, Zhang H, Fan X, Liu K, Zeng D, Tan H. Method Development and validation of indaziflam and its five metabolites in soil, water and fruits by modified QuEChERS and UHPLC-MS/MS. J Agric Food Chem 66(39), 10300–10308 (2018). [CrossRef]
- Mondal R, Mukherjee A, Biswas S, Kole RK. GC-MS/MS determination and ecological risk assessment of pesticides in aquatic system: a case study in Hooghly River basin in West Bengal, India. Chemosphere 206, 217–230 (2018). [CrossRef]
- Kaczynskì P, Lozowicka B, Jankowska M, Hrynko I. Rapid determination of acid herbicides in soil by liquid chromatography with tandem mass spectrometry detection based on dispersive solid phase extraction. Talanta 152, 127–136 (2016). [CrossRef]
- Carvallo D, Moltò JC, Berrada H, Ferrer E. Presence of mycotoxins in ready-to-eat food and subsequent risk assessment, Food Chem Toxicol 121, 558–565 (2018). [CrossRef]
- Zhao H, Chen X, Shen C, Qu B. Determination of 16 mycotoxins in vegetable oils using a QuEChERS method combined with high-performance liquid chromatography-tandem mass spectrometry. Food Addit Contam 34(2), 255–264 (2017). [CrossRef]
- Wei D, Wu X, Xu J, Dong F, Liu X, Zheng Y, Ji M. Determination of Ochratoxin A contamination in grapes, processed grape products and animal-derived product using ultra-performance liquid chromatography- tandem mass spectrometry system. Sci Rep 8(1), 2051–2059 (2018). [CrossRef]
- Durovic-Pejcev RD, Bursic VP, Zeremski TM. Comparison of QuEChERS with traditional sample preparation methods in the determination of multiclass pesticides in soil. J AOAC Int 102, 46-51 (2019). [CrossRef]
- Chen X, Bian Y, Liu F, Teng P, Sun P. Comparison of micellar extraction combined with ionic liquid-based vortex-assisted liquid-liquid microextraction and modified quick,easy,cheap,effective,rugged and safe method for the determination of difenoconazole in cowpea. J Chromatogr A 1518, 1-7 (2017). [CrossRef]
- Khaled A, Singh V, Pawliszyn J. Comparison of solid-phase microextraction to solvent extraction and QuEChERS for quantitative analysis of veterinary drug residues in chicken and beef matrices. J Agric Food Chem 67, 12663-12669 (2019). [CrossRef]
- Sweeney C, Park Y, Kim JS. Comparison of sample preparation approaches and validation of an extraction method for nitrosatable pesticides and metabolites in human serum and urine analyzed by liquid chromatography-orbital ion trap mass spectrometry. J Chromatogr A 1603, 83-91 (2019). [CrossRef]
- Van Der Wat L, Forbes PBC. Comparison of extraction techniques for polycyclic aromatic hydrocarbons from lichen biomonitors. Environ Sci Pollut Res 26, 11179-11190 (2019). [CrossRef]
- Sadilek J, Spalovska P, Vrana B, Vavrova M, Marsalek B, Simek Z. Comparison of extraction techniques for isolation of steroids oestrogens in environmentally relevant concentrations from sediments. Int J Environ Anal Chem 96, 1022-1037 (2016). [CrossRef]
- Tomasini D, Sampaio MRF, Cardoso LV, Caldas SS, Primel EG. Comparison of dispersive liquid-liquid microextraction and the modified QuEChERS method for the determination of fipronil in honey by high performance liquid chromatography with diode-array detection. Anal Methods 3, 1893-1900 (2011). [CrossRef]
- Dusek M, Jandovska V, Kalachova K, Olsovska J. Comparative study of three sample preparation methods for multi-residue extraction of pesticide residues in hop samples. Food Anal Methods 13, 503-515 (2020). [CrossRef]
All site content, except where otherwise noted, is licensed under a Creative Commons Attribution 4.0 License