OPEN-ACCESS PEER-REVIEWED
RESEARCH ARTICLE
Mark J. Perkins1 and Vaughan S. Langford2,*
1Anatune Limited, Wellbrook Court, Girton Road, Cambridge, CB3 0NA, United Kingdom, 2Syft Technologies Limited, 68 Saint Asaph Street, Christchurch 8011, New Zealand.
Reviews in Separation Sciences. Vol. 3. No.2. pages e21003 (2021).
Published on-line 15 May 2021. https://doi.org/10.17145/rss.21.003 | (ISSN 2589-1677).
- Abstract
- Keywords
- 1.0 Introduction
- 2.0 SIFT-MS and its Automation for Routine Analysis
- 3.0. SIFT-MS Method Development
- 4.0 Method Transfer and Validation
- Figures and Tables
- 5.0 Routine Analysis Workflow
- 6.0 Applying Quantitation Approaches used with Chromatograpic Methods
- 7.0 Simplified and novel routine analysis with SIFT-MS
- 8.0 Conclusion
- 9.0. References
*Correspondence:
Langford VS and Perkins MJ .
Syft Technologies Limited, 68 Saint Asaph Street, Christchurch 8011, New Zealand & Anatune Limited, Wellbrook Court, Girton Road, Cambridge, CB3 0NA, United Kingdom.
Editor: Mark Shapiro, MCS Pharma Consulting LLC, Stokesdale, NC 27357, USA..
Open-access and Copyright:
©2021 Perkins MJ and Langford VS. This article is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Funding/Manuscript writing assistance:
The authors declare that no funding and writing assistance was utilized in the production of this article.
Competing interest:
The authors are employees of affiliated companies, they have declared that no competing interest exist.
Article history:
Received 04 March 2021, Revised 21 April 2021, Accepted 22 April 2021.
Abstract
In recent years the environmental and human health impacts of volatile organic compounds (VOCs) have become more apparent, resulting in increased analysis demand. The gold-standard chromatographic techniques continue to be employed for most laboratory analyses. However, they have made only modest gains in productivity over the years, and these gains are primarily due to automated sample preparation and injection. Alternatively, significant productivity gains for VOC analysis through faster sample analysis and reduced instrument maintenance could be achieved by adopting direct mass spectrometry techniques such as selected ion flow tube mass spectrometry (SIFT-MS). This article demonstrates that routine analysis techniques such as quality control checks, method validation, the method of standard additions, and internal standards are readily applied to SIFT-MS, simplifying adoption of the technique. In addition, workflows for analysis of chromatographically challenging species are simplified by using SIFT-MS. Sample throughputs are increased two- to 25-fold depending on the analytical procedure.
Keywords
SIFT-MS, headspace analysis, method validation, soil, water, air, volatile compound.
1.0 Introduction
Chromatographic methods have been the mainstay of the volatile organic compound (VOC) analyses conducted by routine laboratories and contract research organizations (CROs) for decades. Gas chromatography (GC) methods (with various detector options) are the most widely employed for analysis of VOCs. Examples include the United States Environmental Protection Agency (US EPA) methods for environmental monitoring [1-4], US NIOSH methods for workplace exposure to VOCs [5,6], International Standards Organization (ISO) methods for indoor air quality [7,8], and United States Pharmacopeia (USP) methods such as that for residual solvents [9]. Liquid chromatography (LC) mainly finds application in analysis of semi- and non-volatile organics (especially for biomolecules) [10], but is sometimes applied to VOCs–especially those of higher polarity. An example is the analysis of low molecular weight aldehydes (following derivatization) using high-performance liquid chromatography (HPLC) in the widely used US EPA Method TO-11A [11].
As demand for routine VOC analyses increases, sample throughputs on a given analytical system are not increasing proportionately because the chromatographic separation that underpins GC and HPLC methods remains the most significant limitation on maximum throughput. Depending on the analytical method, typical chromatographic analyses range from 10 to 50 minutes each. A solution that would shorten analysis times could be achieved by adoption of direct mass spectrometry (DMS) into routine analysis workflows at a minimum to provide rapid screening. DMS methods specifically developed for VOC analysis include atmospheric pressure chemical ionization-MS (APCI-MS) [12], proton transfer reaction-MS (PTR-MS) [13,14], and selected ion flow tube MS (SIFT-MS) [15,16]. Although all techniques date from the 1990s and a significant body of research has developed for each [17], only SIFT-MS is currently a serious contender for routine analysis alongside the chromatographic methods. Compared to PTR-MS and APCI-MS, the combination of very highly controlled soft chemical ionization (enabling a compound library to be provided for both identification and quantification), rapid reagent ion switching, and stable quantitation, make SIFT-MS the best-suited technique for the routine analysis laboratory [17,18].
However, for those investigators new to direct MS who are considering its adoption into routine analysis or a contract research organization (CRO), some adaptation of routine techniques is required.
This “how-to” article reviews work conducted since 2015 to apply common routine analysis procedures developed for separation-based analytical approaches to direct-injection analysis using SIFT-MS. First, it introduces the SIFT-MS technique and its automation. Second, it considers the nuances of method development, method transfer, and method validation as applied to SIFT-MS. Then it describes how SIFT-MS fits comfortably into routine analysis workflows and can have chromatographic quantitation approaches applied to it. The article concludes with a discussion of how SIFT-MS can also simplify the analysis of chromatographically challenging compounds across diverse [19-23] and novel [24-28] applications.
2.0 SIFT-MS and its Automation for Routine Analysis
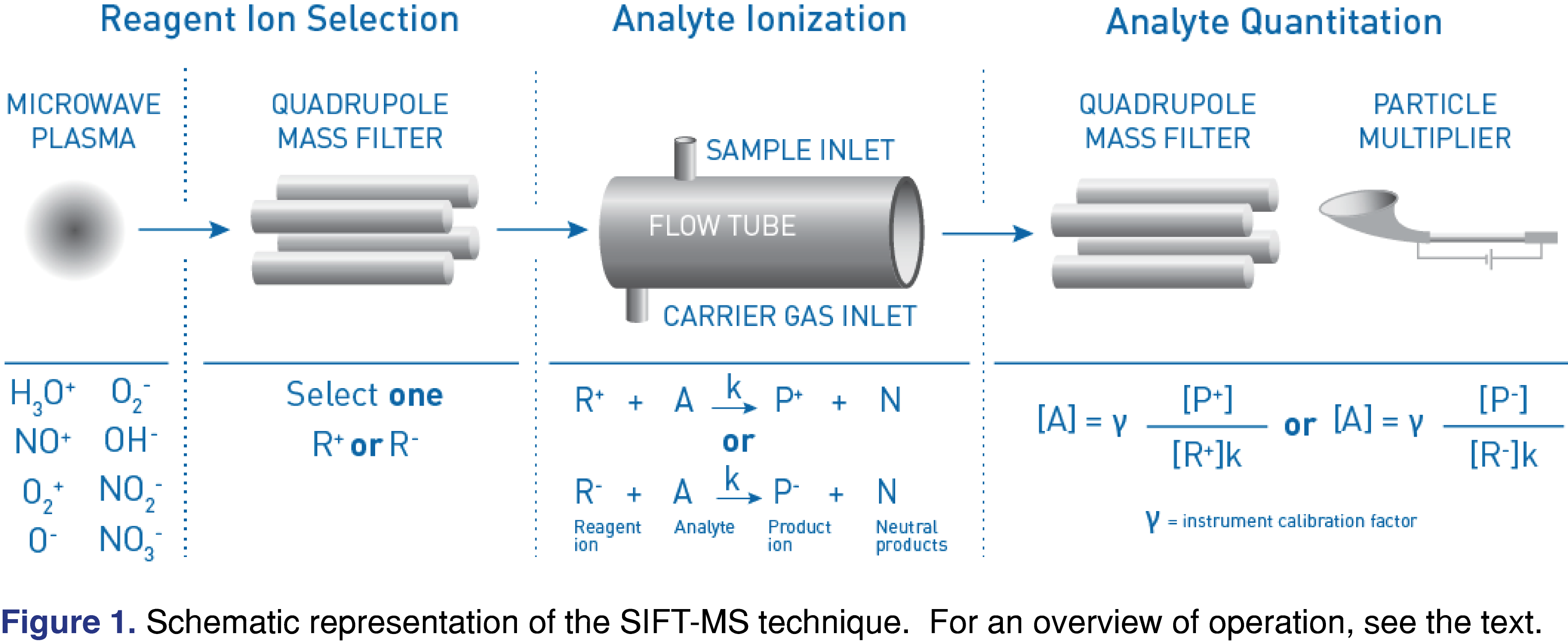
SIFT-MS is an emerging, direct mass spectrometric analytical technique that is well suited to the routine analysis laboratory. The SIFT-MS technique itself has been described in detail elsewhere [15-18]. Briefly, SIFT-MS uses controlled, soft chemical ionization coupled with mass spectrometric detection (Figure 1) to rapidly quantify VOCs in air and headspace to part-per-trillion concentrations by volume (pptV). Up to eight chemical ionization agents (reagent ions) are available in commercial SIFT-MS instruments (H3O+, NO+, O2+, O–, O2–, OH–, NO2-, and NO3-) [20], but the positive ions are the standard configuration. The reagent ions react with VOCs and inorganic gases in well-controlled ion-molecule reactions, but they do not react with the major components of air (e.g. N2, O2, and Ar) and only slowly with water, facilitating trace analysis without pre-concentration or drying of samples. Rapid switching of reagent ions using a quadrupole mass filter provides high selectivity in the absence of chromatographic pre-separation. Data obtained here utilized a Syft Technologies Voice200ultra SIFT-MS instrument (Syft Technologies, Christchurch, New Zealand; www.syft.com). The units presented for headspace concentration determinations using SIFT-MS are parts-per-million by volume (ppmV). Although volume units are the “natural” units for SIFT-MS quantification indirect gas phase analysis, in the context of headspace they can also be viewed as a response factor from which concentrations in solution can be derived via a calibration curve. SIFT-MS headspace analysis is automated using a syringe-injection autosampler (for example, the Multipurpose Sampler (MPS) from GERSTEL, Mülheim an der Ruhr, Germany; www.gerstel.com), which provides slow, precisely controlled injection for the duration of the analysis. Syringe-injection autosamplers are essential for direct MS techniques such as SIFT-MS because–in contrast to chromatographic methods–sample analysis occurs synchronously with sample introduction (Figure 2) [29]. Headspace analysis is most commonly conducted from 20 mL sample vials on a standard GERSTEL vial rack.
3.0. SIFT-MS Method Development
Direct sample analysis without chromatographic separation introduces some significant differences for SIFT-MS method development compared to conventional chromatographic techniques. It is outside the scope of this article to discuss the details here, so the key differences are summarized.
3.1 Matrix Effects
Since the SIFT-MS technique has no chromatographic column to separate solvents and other dominant matrix species from the analytes, the total load of reactive compound in samples needs to be considered. If samples need to be diluted to avoid overloading the SIFT-MS instrument (i.e. to keep it within its dynamic range), then the LOQs of target compounds will be affected proportionally. An example of coping with the matrix is summarized below in a case study where a methanolic extraction method developed for GC/MS is transferred to SIFT-MS.
3.2 Specificity
Since SIFT-MS does not resolve compounds chromatographically, selectivity is achieved by using a combination of chemical separation (provided by multiple soft chemical ionization agents) and mass spectrometric detection [17,18]. Software tools assist the user with resolving compounds [18]. Additionally, a calibration approach [30,31] sometimes aids resolution of certain isomeric species – in this approach, ethylbenzene is distinguished from the measurement of total xylenes based on different fragmentation patterns with the O2+ reagent ion [32].
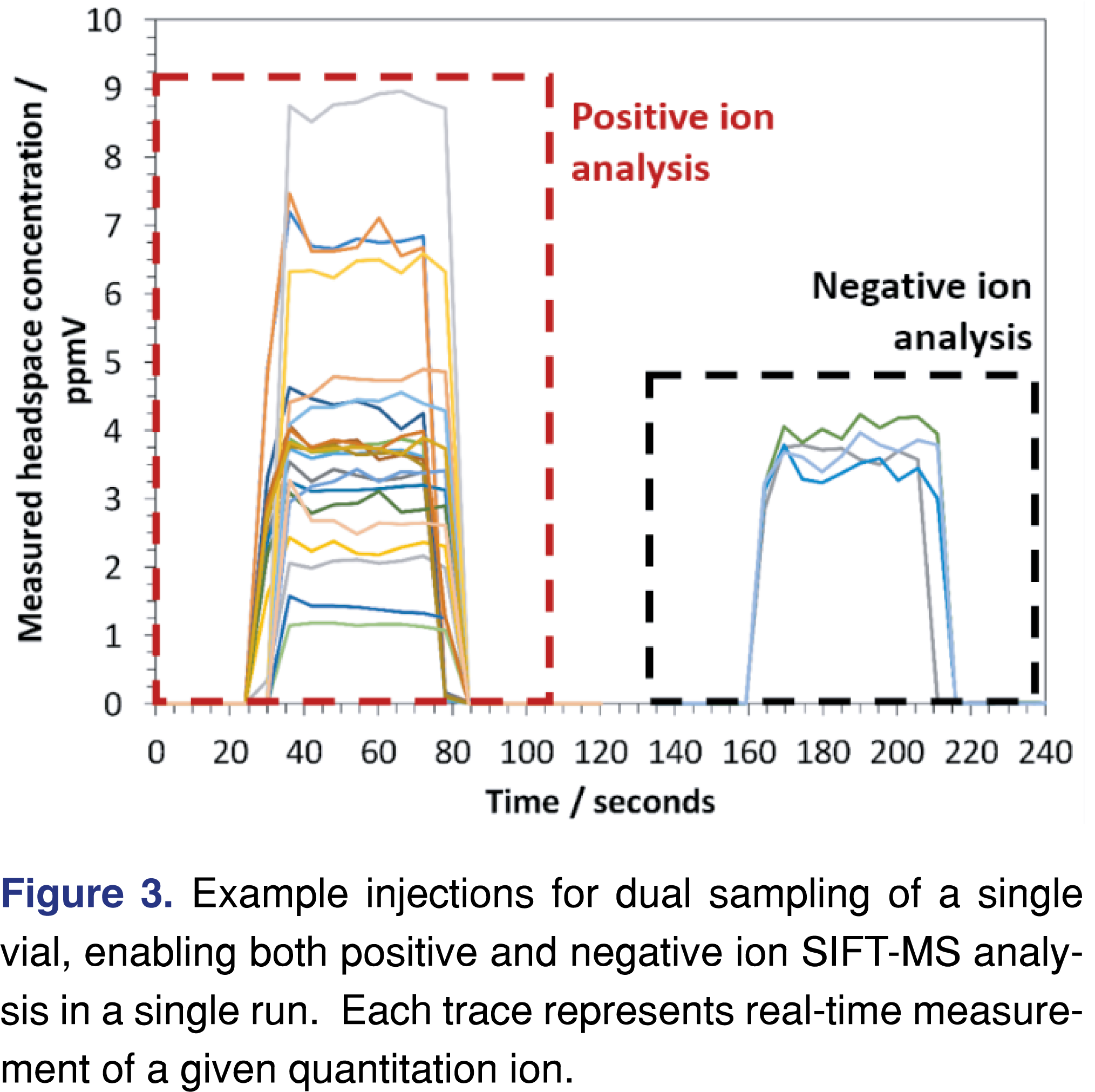
If both positively and negatively charged reagent ions are utilized, the current commercial option is unable to instantly switch polarities (the switch takes about 30 seconds due to a change in ion source pressure, because higher pressures are required to form negative reagent ions through the electron attachment process). However, with automation, the preparation of duplicate samples is obviated: duplicate or dual sampling of each sample vial is readily undertaken. A recent study [31] has successfully applied and validated dual sampling of single vials for the first time with SIFT-MS. Data from a dual-sampling analytical approach is shown in Figure 3, where all positive quantitation ions are analyzed on the first injection and negative ions on the second. For static headspace analysis, this approach yields a throughput of about 180 samples per day, compared with approximately 280 per day with single sampling and reduced specificity. For comparison, the Anatune “VOC analyzer” has a throughput of 97 samples per day [33].
3.3 Limits of Detection and Quantitation
In SIFT-MS, the LODs and LOQs are improved by increasing one or more of (i) reagent ion signal, (ii) flow of sample into the flow tube, and (iii) the measurement time for target compounds [34], which contribute to improved measurement statistics. This means that limitations may be imposed on the number of compounds that can be analyzed to the required LOQ or LOD for a given sample injection due to the limited sample volume of the headspace syringe coupled with the injection rate into the SIFT-MS instrument (see, for example [23]). This contrasts with chromatographic methods, where higher sensitivities and hence improved LODs and LOQs are obtained by injecting more sample on the column [35].
3.4 Sample Delivery
As described elsewhere [29], the direct sample analysis in SIFT-MS requires that sample be introduced continuously and steadily while the instrument is analyzing the sample. As noted above, automated SIFT-MS headspace analysis is most readily achieved using autosamplers based on the syringe-injection technique, and the injection window is averaged to determine the headspace concentration, since it is constant throughout the injection (Figure 3). For thermal desorption or thermal extraction analyses using SIFT-MS detection, a constant desorption flow rate is utilized, but data are integrated to determine the concentration due to the time-dependent nature of the desorption profile.
4.0 Method Transfer and Validation
The direct analysis inherent to SIFT-MS yields increased sample throughputs compared to chromatographic methods. Hence, when the matrix is compatible with SIFT-MS and the target compounds are amenable to detection, method transfer from chromatographic techniques to SIFT-MS may be feasible. If the method is automated for chromatographic analysis, then the transfer to SIFT-MS is likely to be straightforward, as demonstrated in the case study below. Validation of SIFT-MS methods is achieved using an approach developed from the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) guideline document Validation of Analytical Procedures: Text and Methodology Q2(R1) [36] as demonstrated elsewhere for gas [37] and headspace [31] analyses.
Figures and Tables
[Click to enlarge]
4.1 Case study: Methanolic Extraction of BTEX from Soil
Methanolic extraction is a widely used preparation technique for the analysis of benzene, toluene, ethylbenzene, and xylene (BTEX) in contaminated soils [38,39]. The two most used sample introduction techniques for GC/MS are closed-system purge-and-trap and static headspace. Single quadrupole GC/MS is the main chromatographic and detection method, but all conventional methods have drawbacks. It has been shown previously that the headspace methodology can be automated using the GERSTEL MPS sampler on GC/MS [40]. Whilst the sample preparation time remains the same in transferring the method to SIFT-MS, the analysis time is reduced threefold. Full details on the sample preparation and the results obtained are provided elsewhere [41] so a summary of results is provided here.
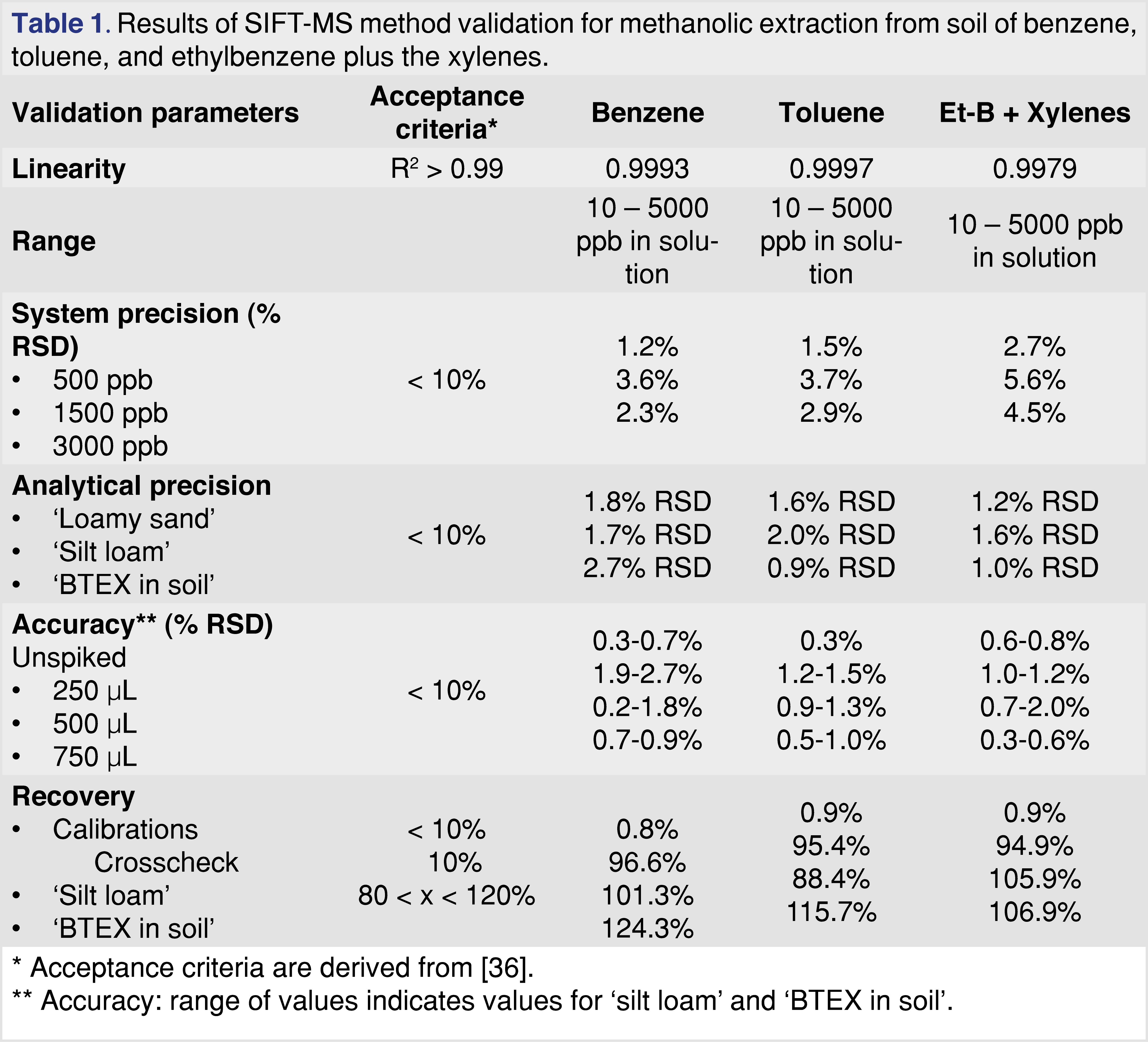
There were several factors to consider in transferring this method to SIFT-MS. First, the methanol used to extract the VOC residues from soil reacts rapidly with both the H3O+ and O2+ reagent ions, but about 100-fold slower with NO+ [42] This means that – due to residual methanol in headspace samples – only the NO+ reagent ion can be utilized. This is entirely satisfactory for benzene and toluene, but it means that ethylbenzene cannot be resolved from the xylenes using the O2+ reagent ion with the approach described elsewhere [30,31]. Second, more contaminated samples may cause the linear range of the instrument to be exceeded when a 2.0-g soil sample is used. Linear response was observed over the evaluated range from 0.25 g to 2.0 g, providing flexibility to the analyst in handling more contaminated soils. Finally, it is possible that residual extraction solvent could overwhelm the NO+ reagent ion. Hence the effect of different extract spike volumes (from 25 to 500 µL) was evaluated, also giving a linear response.
Validation of the transferred method was conducted using the approach described elsewhere [31]. The results of validation are summarized in Table 1 with full data in [41]. Three soil samples (proficiency test samples ‘loamy sand’, ‘silt loam’, and ‘BTEX in soil’ from Sigma-Aldrich, Gillingham, UK) were used to determine analytical precision. Recovery was evaluated in triplicate on the ‘silt loam’ and ‘BTEX in soil’ samples. Except for several ‘BTEX in soil’ samples over-recovering, all acceptance criteria (from [36]) were met.
5.0 Routine Analysis Workflow
In this section, recommended procedures for implementing routine calibration, system suitability tests, and quality control checks are discussed.
5.1 Calibration Approaches for SIFT-MS
Regular calibration of SIFT-MS instrumentation is a departure from the conventional SIFT-MS approach, where the quantitation based on pseudo-first-order gas-phase reaction kinetics [15,16] and instrument stability deem calibration uncommon. However, calibration is simple and rapid for automated SIFT-MS instruments, so transferring routine chromatography calibration procedures to the SIFT-MS workflow is recommended.
Conventional applications of SIFT-MS instruments frequently involve continuous, real-time analysis. These have utilized gas-phase calibration from gas calibration standards (cylinders and permeation tubes being commonly used). In contrast, automation greatly simplifies calibration, enabling more cost-effective, easy-to-handle solution-based standards to be utilized. The most important consideration is that because SIFT-MS is a direct analysis method, criteria determining solvent compatibility differ from GC/MS. For SIFT-MS, the solvent must be non-reactive or react only very slowly with one or more SIFT-MS reagent ions. In contrast to GC/MS, water is the preferred solvent for SIFT-MS. However, in various applications methanol (see the case study in the preceding section), chloroform, dichloromethane, and dimethylsulfoxide (DMSO) [31,43] have all been utilized successfully.
Calibration standards can be prepared automatically on the autosampler platform if a compatible model is used (for example, the GERSTEL MultiPurpose Sampler (MPS) Robotic-Pro with tool change, or the GERSTEL dual-head MPS XT). If either GERSTEL platform is used, then the GERSTEL Maestro PrepAhead functionality enables this preparative work to be done very efficiently, in addition to the calibration sequence itself.
Analogous to GC/MS, multiple-point calibration curves covering the range of the analysis and single-point calibrations are both supported by automated SIFT-MS.
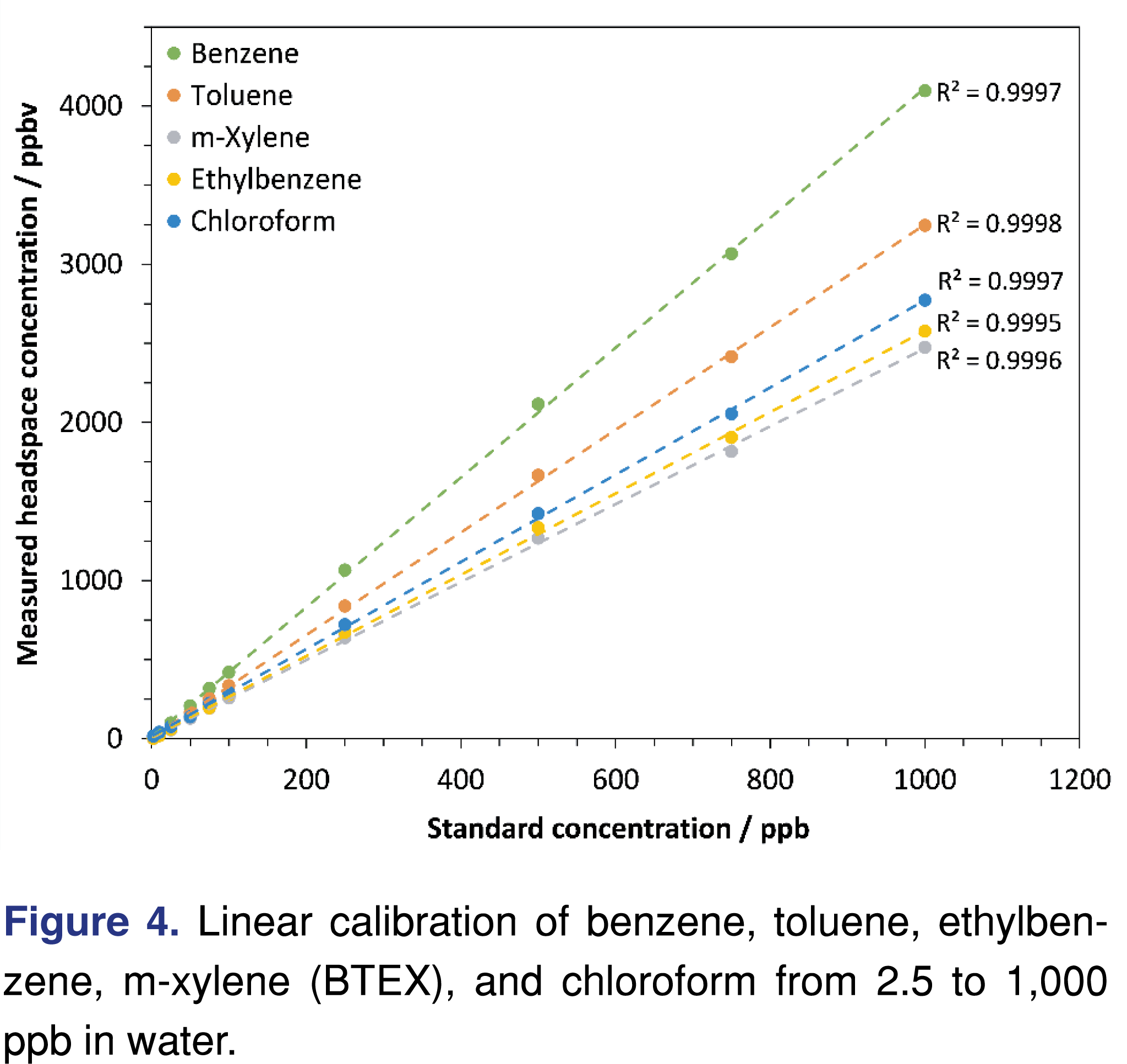
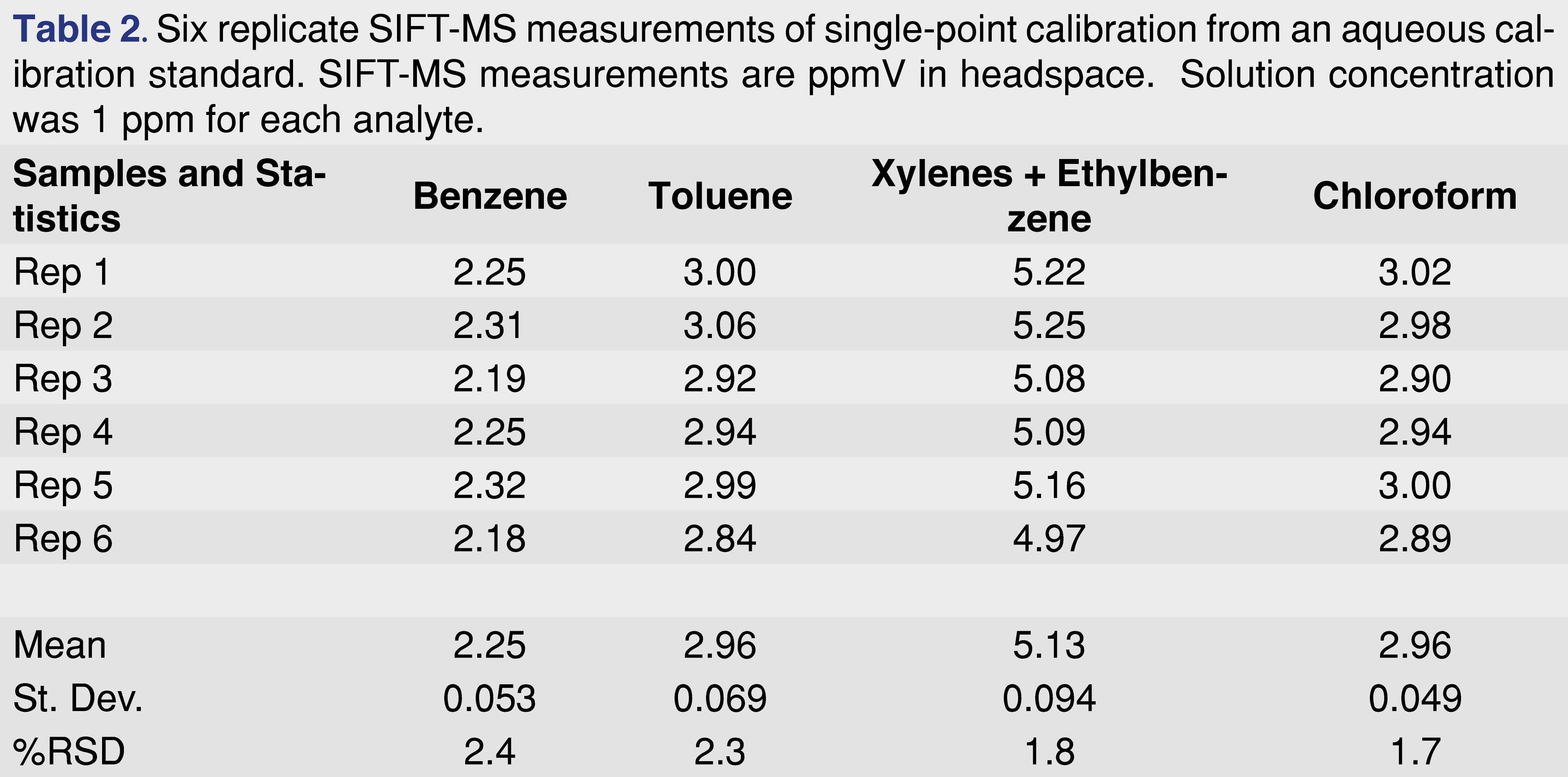
Multiple-point calibrations. Multiple-point calibrations have been implemented regularly in automated SIFT-MS analyses. Figure 4 shows an example for BTEX and chloroform analysis in the headspace of water at 60°C, demonstrating the excellent linearity of aqueous headspace analysis using automated SIFT-MS. Similar results were obtained for soil headspace analysis (described above) and gas-phase analysis [37,44].
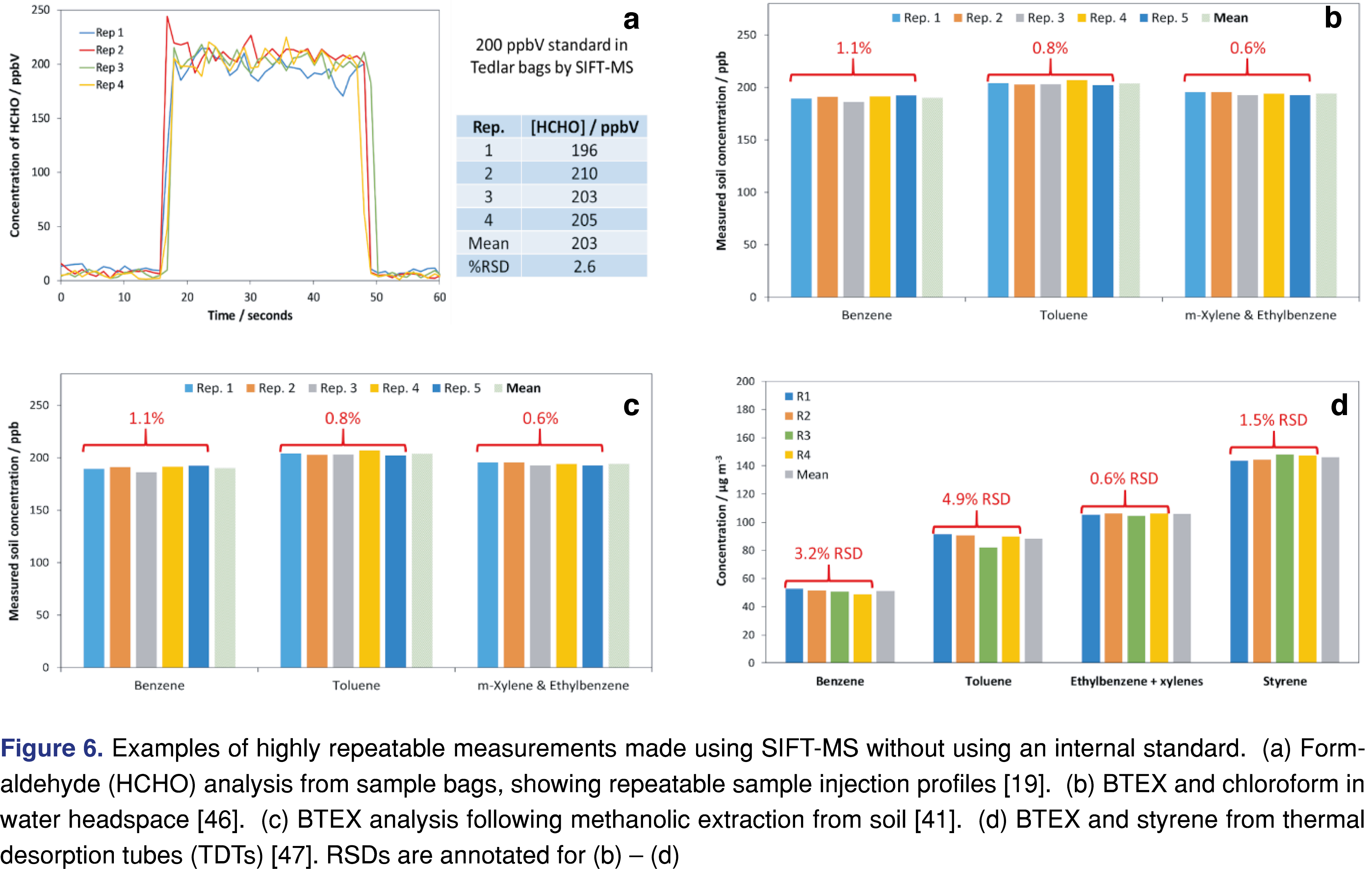
Single-point calibrations. Automated SIFT-MS provides extremely repeatable analysis, even for headspace measurements when no internal standard is utilized (see ‘Internal Standards for SIFT-MS’ below). This means that single-point calibration is justified for certain methods. Table 2 summarizes repeatability for six water headspace measurements; all analytes have RSDs less than 2.5%.
5.2 System Suitability Tests for SIFT-MS
System suitability tests (SSTs) verify that the analytical system will perform in accordance with the criteria set forth in the method [9]. SSTs are performed along with the sample analyses to ensure that the system’s performance is acceptable at the time of the test. Here, an example SST for SIFT-MS is presented that was utilized in a regulatory submission for formaldehyde analysis in novel drug delivery devices.
The manufacturer of the SIFT-MS instruments utilized in this work recommends running an automated daily qualification routine on their SIFT-MS instrument(s) that utilizes an eight-component certified gas mixture [45]. (This can be considered equivalent to the autotune procedure run on a GC/MS.) This gas standard is utilized here for a generic SST.
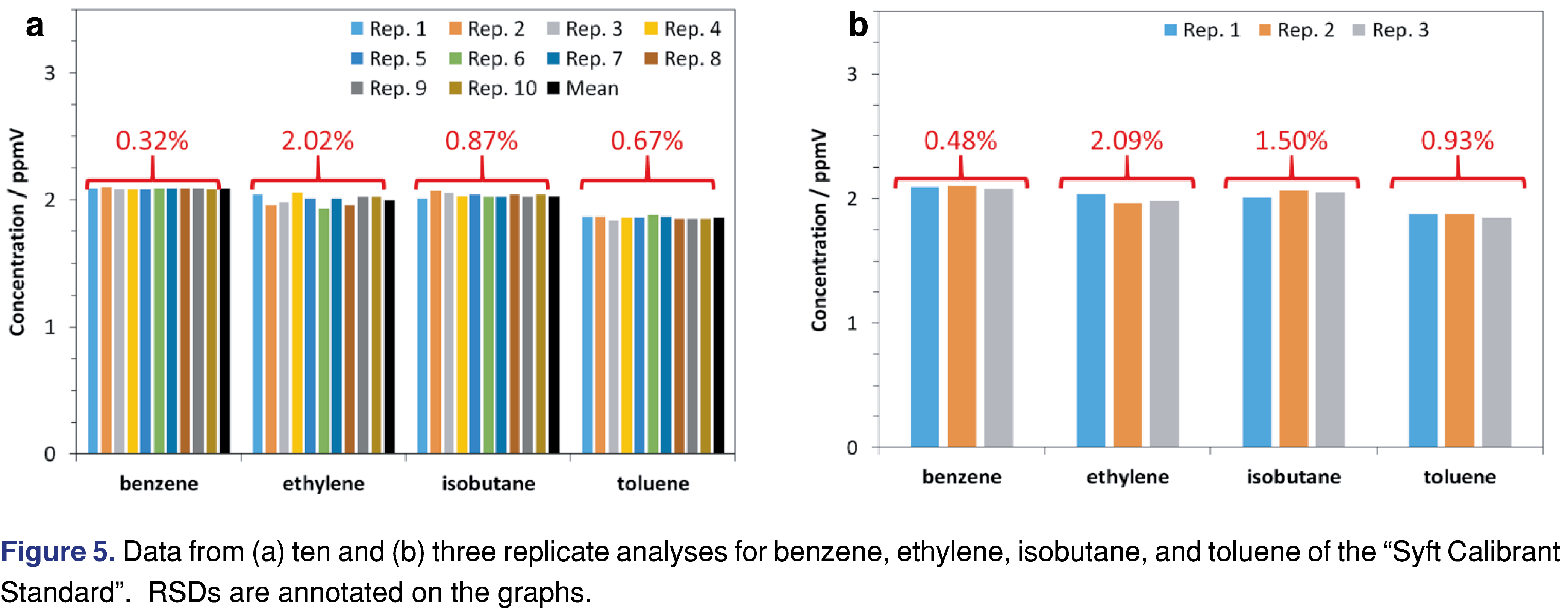
After running the full daily qualification routine, the quantitation test was performed in replicate several times. In this example, it was run 10 times and four compounds (benzene, ethylene, isobutane, and toluene) were used to assess performance. These replicate measurements took less than seven minutes to obtain. Figure 5(a) shows the replicates and means for 10 replicates, with RSDs of ca. 2% or less shown in red. However, this rapid SST can be further reduced to little more than two minutes by simply making triplicate measurements Figure 5(b), since RSDs are still well within typical acceptance criteria [36]. Following the principles described for developing a generic SST, rapid SSTs for specific methods can be created. In the context of this regulatory submission [37], a rapid specific SST was developed in which formaldehyde was analyzed from a permeation tube standard.
5.3 Quality Control Checks for SIFT-MS
Some methods require inclusion of quality control check (QCC) standards to provide within-sequence assurance that the method continues to perform properly. Analytical instrument qualification, analytical method validation, and SSTs contribute to quality assurance of the analysis before samples are analyzed. The QCCs assure quality analytical results are obtained during the sample analysis sequence.
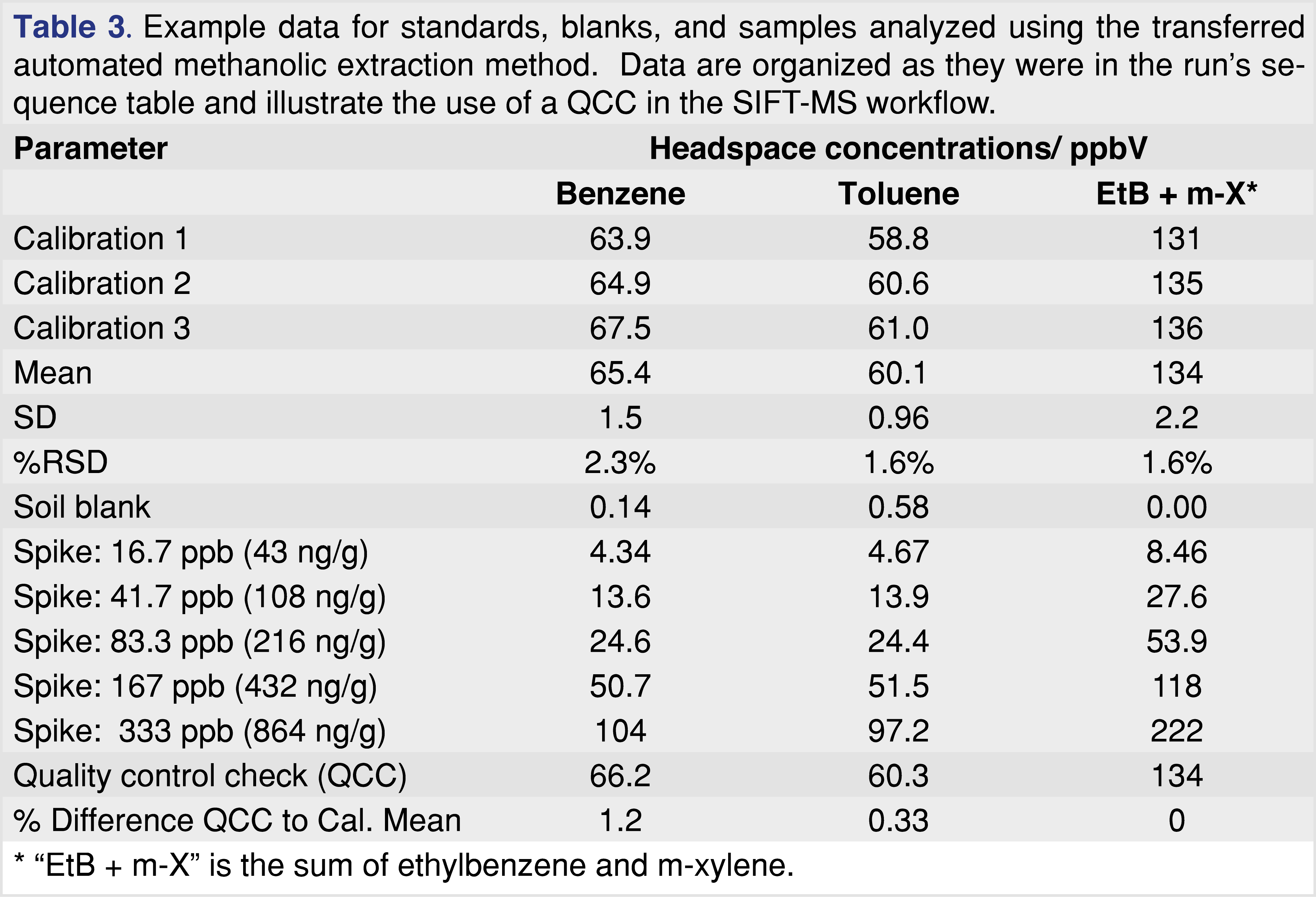
QCCs are easily implemented and the samples are rapidly analyzed with automated SIFT-MS. Table 3 summarizes results obtained from a short demonstration sequence for methanolic extraction of BTEX from soil (summarized in the previous section and described fully in [41]). The QCC results all lie within 1.2% of the original triplicate calibration, so acceptance criteria are met [36].
The rapid analysis provided by SIFT-MS means that more QCCs can be added in the sequence and additional QA checks can be made. For example, regular interleaving of blanks ensures that carryover is within acceptable limits.
6.0 Applying Quantitation Approaches used with Chromatograpic Methods
For certain methods, routine calibration has proved inadequate for chromatographic methods, so internal standards and/or the method of standard additions are incorporated. In this section, SIFT-MS approaches to applying these procedures are described.
6.1 Internal Standards for SIFT-MS Analysis
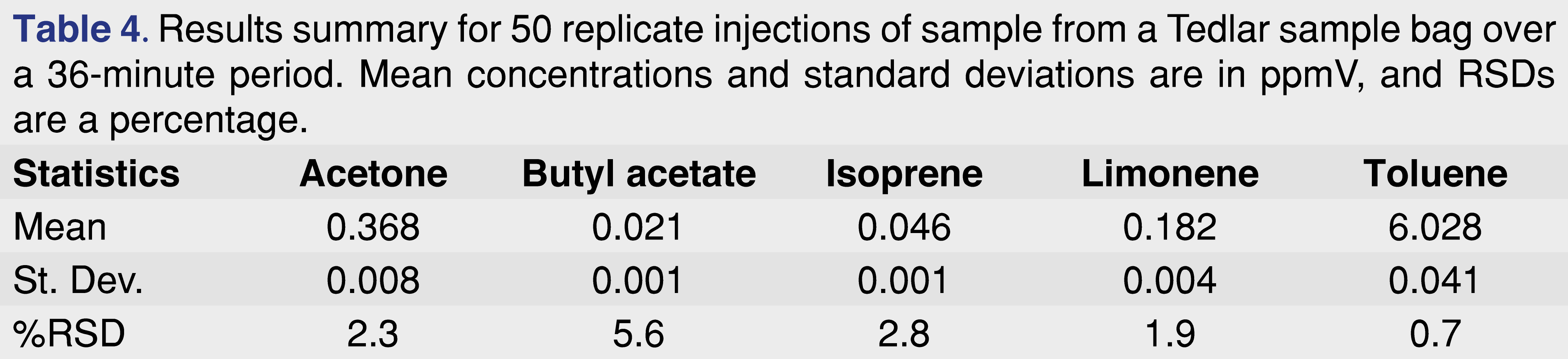
For most applications of automated SIFT-MS, the use of internal standards has been unnecessary (cf. Figure 4 for aqueous headspace analysis with SIFT-MS). In contrast to headspace-GC/MS, where there is significant variability and internal standards are essential, headspace-SIFT-MS measurements are routinely very precise (Figure 6). It appears that the reason for this difference is that the steady, slow injection into the sample inlet of the SIFT-MS instrument yields much less variability in headspace-SIFT-MS (see Table 4 for the results summary for 50 replicate injections from a Tedlar bag sample). In contrast, for headspace-GC/MS, a relatively large headspace volume is injected into a small liner volume very rapidly, likely introducing greater variability.
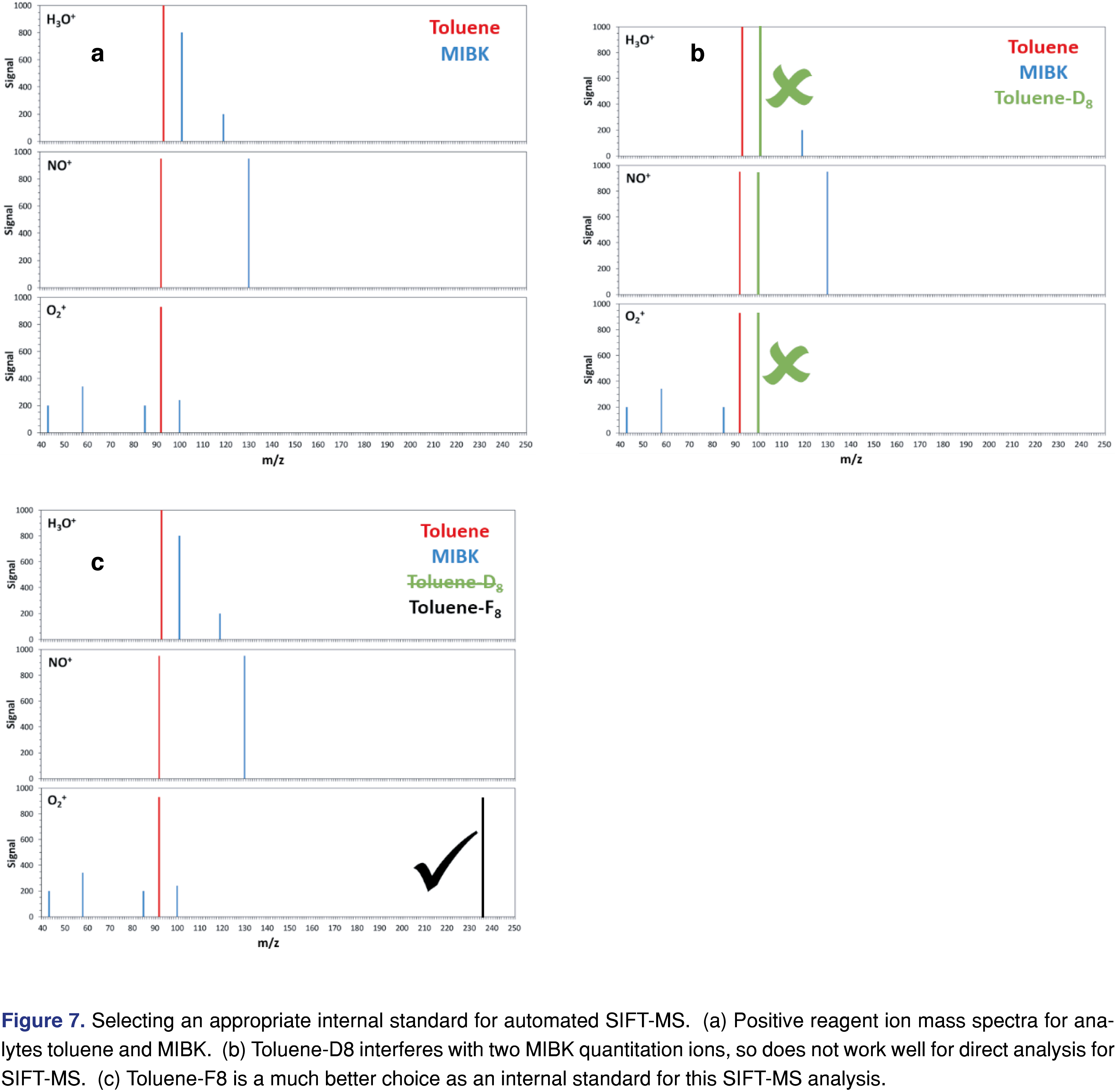
Although SIFT-MS methods do not ordinarily require internal standards, the procedure is readily applied to SIFT-MS – albeit with some modifications compared to GC/MS. Because SIFT-MS analysis has no chromatographic separation, application of the internal standards used in chromatographic methods (e.g. deuterated standards) may not give good results for SIFT-MS. Selection of an internal standard for a SIFT-MS method requires that the matrix and the target compound list be evaluated in detail to ensure (1) that the internal standard does not interfere with other analytes, and (2) that the standard is not interfered with by other analytes or the matrix. That is, the internal standard needs to have product ions in regions of the mass spectra where there are no matrix or analyte peaks.
Consider a simple example: the analysis of two industrial solvents: toluene and methyl isobutyl ketone (MIBK). These compounds have molecular weights of 92 and 100 Da respectively. Their SIFT-MS positive reagent ion scan spectra are shown in Figure 7(a). if toluene-D8, a conventional internal standard for GC/MS, is used, then a significant interference issue results with two of the available quantitation ions for MIBK (as shown in Figure 7(b): with H3O+, m/z 101 and with O2+, m/z 100). However, fluorinated internal standards provide a promising alternative. Consider perfluorotoluene (or toluene-F8; Figure 7(c)): its product ion is shown overlaid on the spectrum and cause no problems.
6.2 Case study: Solvents in Plasma
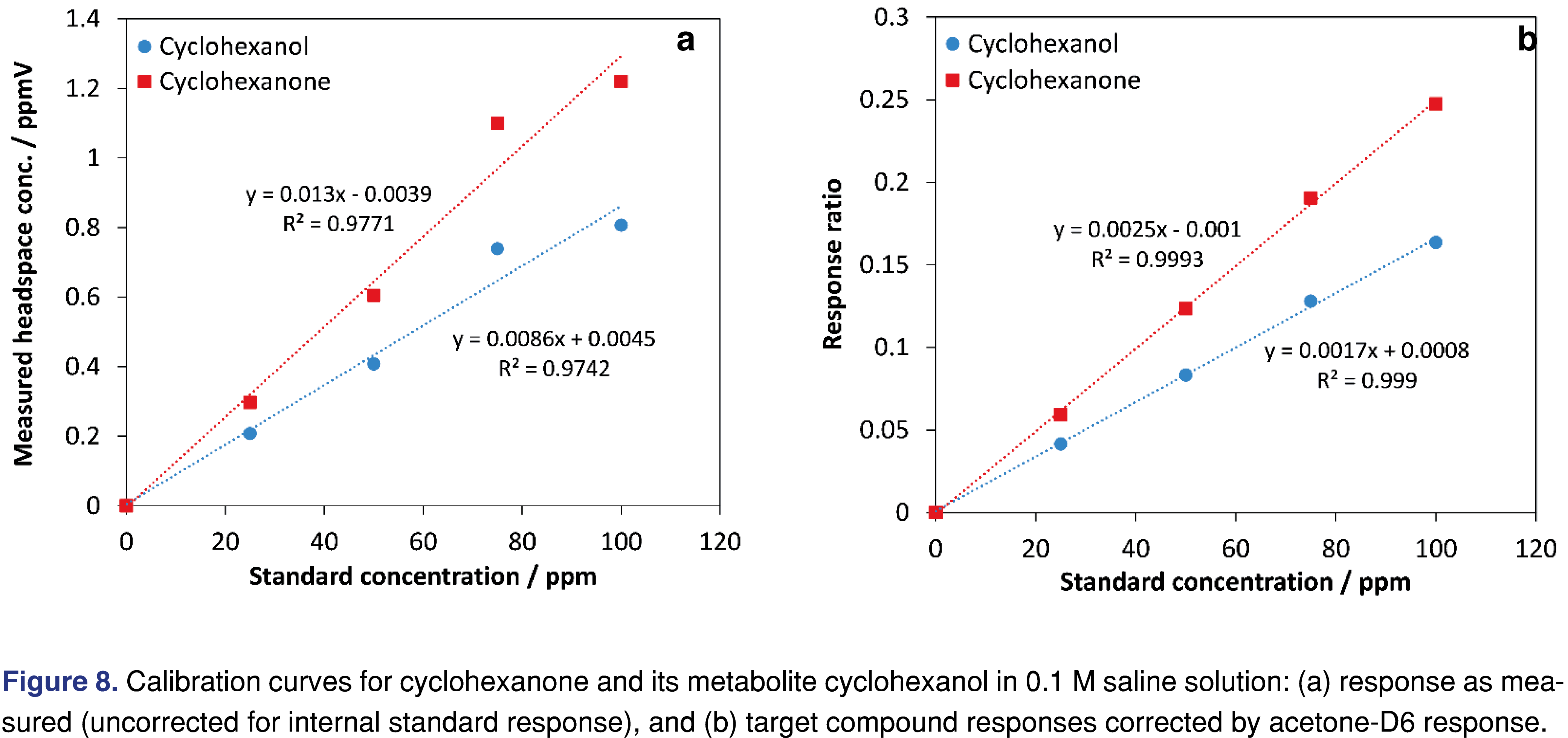
Early method development in a recent study [48] investigating cyclohexanone and its metabolite cyclohexanol in porcine plasma utilized acetone-D6 as an internal standard (100-ppm in solution) due to some variability in the headspace data [49]. This preliminary method development work utilized standards in 0.1 M saline solution, not plasma. Figure 8(a) shows the calibration curves for cyclohexanone and cyclohexanol uncorrected, while Figure 8(b) shows the points corrected by the acetone-D6 internal standard’s response – illustrating a significant improvement. Interestingly, as method development progressed inclusion of an internal standard for the SIFT-MS analysis was deemed unnecessary based on method performance criteria being met without it [48].
6.3 Standard Additions for SIFT-MS
The method of standard additions is really the “gold standard” analytical method, because each sample carries its own calibration curve and thus any drift in the method over time is mitigated [50-52]. However, performing the method of standard additions analysis is expensive due to multiple analyses being conducted per sample and it is typically only utilized when essential. Examples of such usage include when the matrix effects prevent good chromatography, or when the matrix modifies the partitioning of the analyte in headspace analysis. Mitigation of matrix effects has seen it applied in SIFT-MS analysis. Sample throughput using SIFT-MS is reduced compared to routine headspace analysis, but it is still significantly faster than the method of standard additions utilized with GC/MS.
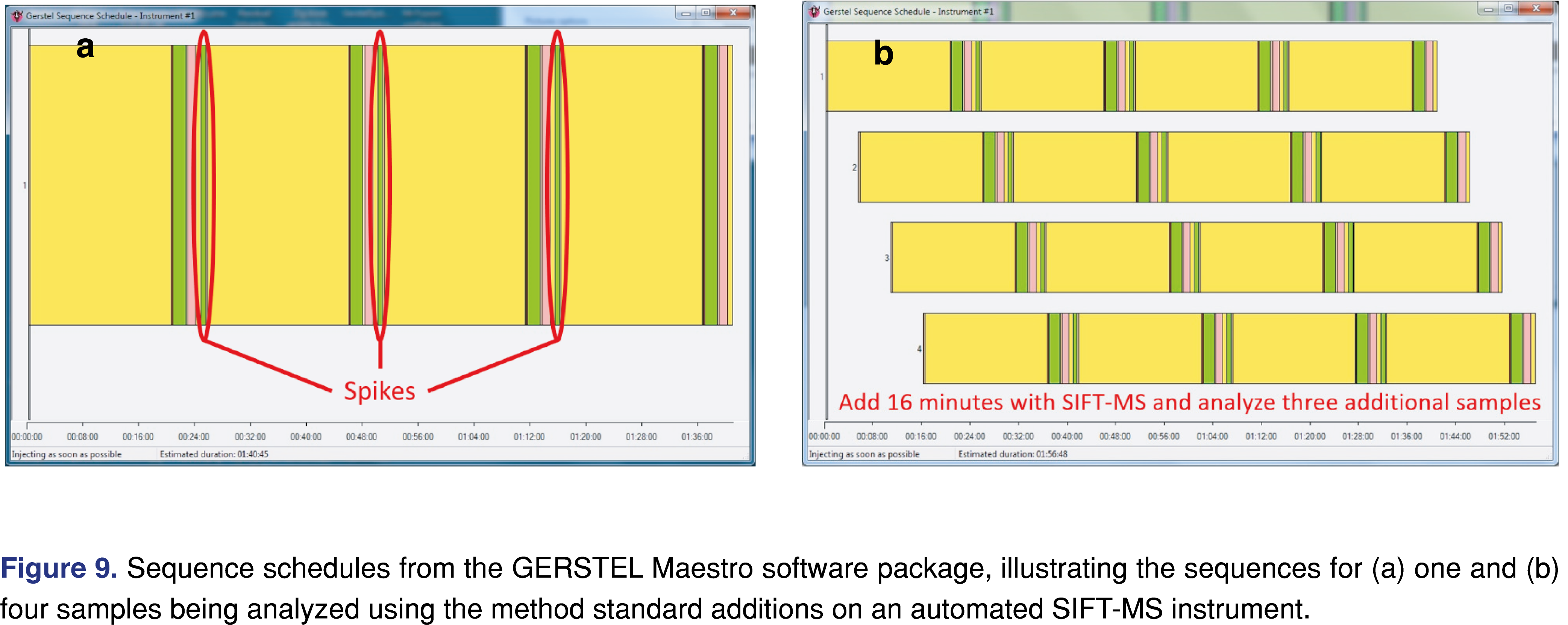
To apply the method of standard additions to SIFT-MS, automation is recommended. When developing the method, the spike volumes of standards should be kept as small as possible so that additional matrix effects are not introduced; typically, this means using 1-10 µL spikes. The method developer should also ensure that headspace partitioning is not perturbed through multiple cycling of spikes and analysis; if perturbation of headspace partitioning is observed, multiple vials per sample should be prepared (one for each spike level). If method development shows that multiple sampling of the headspace from the same vial can be conducted, then the process is (1) incubate, (2) inject, (3) add spike, repeating for the number of standard additions that will be made. For a curve with three spike additions, the sequence for one sample is shown in Figure 9(a). Because the time for analysis is not the rate-limiting step with SIFT-MS (as it is for GC/MS), three additional samples (or if the standard additions samples need to be prepared separately) increase the analysis time by only 16 minutes (or 17%; Figure 9(b)).
6.4 Case study: Formaldehyde Analysis in Fragrance Matrices
Variability in fragrance matrices means that formaldehyde cannot be analyzed reliably using the routine headspace-SIFT-MS approach. Two significant contributors are (1) partitioning of formaldehyde from aqueous solutions is relatively low – resulting in relatively poor sensitivity (~0.1 µg/mL), and (2) high levels of VOCs in the fragrance matrix consumed reagent ion signals but were not consistent from one sample to the next, leading to overestimation of concentration. Hence the method of standard additions was employed to address this.
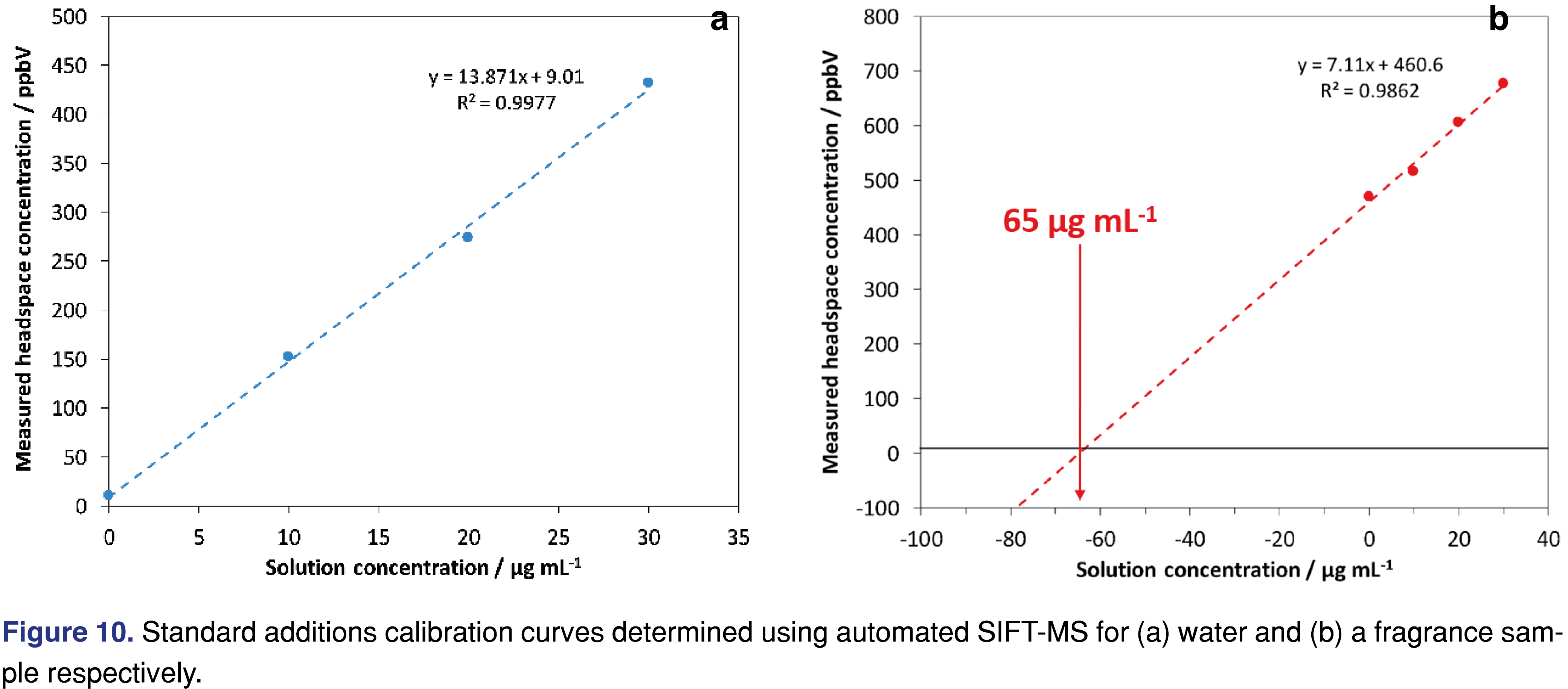
A simple aqueous calibration set for formaldehyde quantitation did not match the complex fragrance matrix adequately, so the method of standard additions was utilized to overcome this issue. In this case study, 1 mL of sample was added to a 20 mL vial and it was incubated for 15 minutes at 75°C. After the headspace was analyzed, standard additions were made at 10, 20 and 30 µg/mL from a 1000 µg/mL standard. Figure 10 shows the linearity across the standard additions range in (a) water and (b) a fragrance sample. For the fragrance sample, extrapolation revealed the presence of 65 µg/L of formaldehyde.
7.0 Simplified and novel routine analysis with SIFT-MS
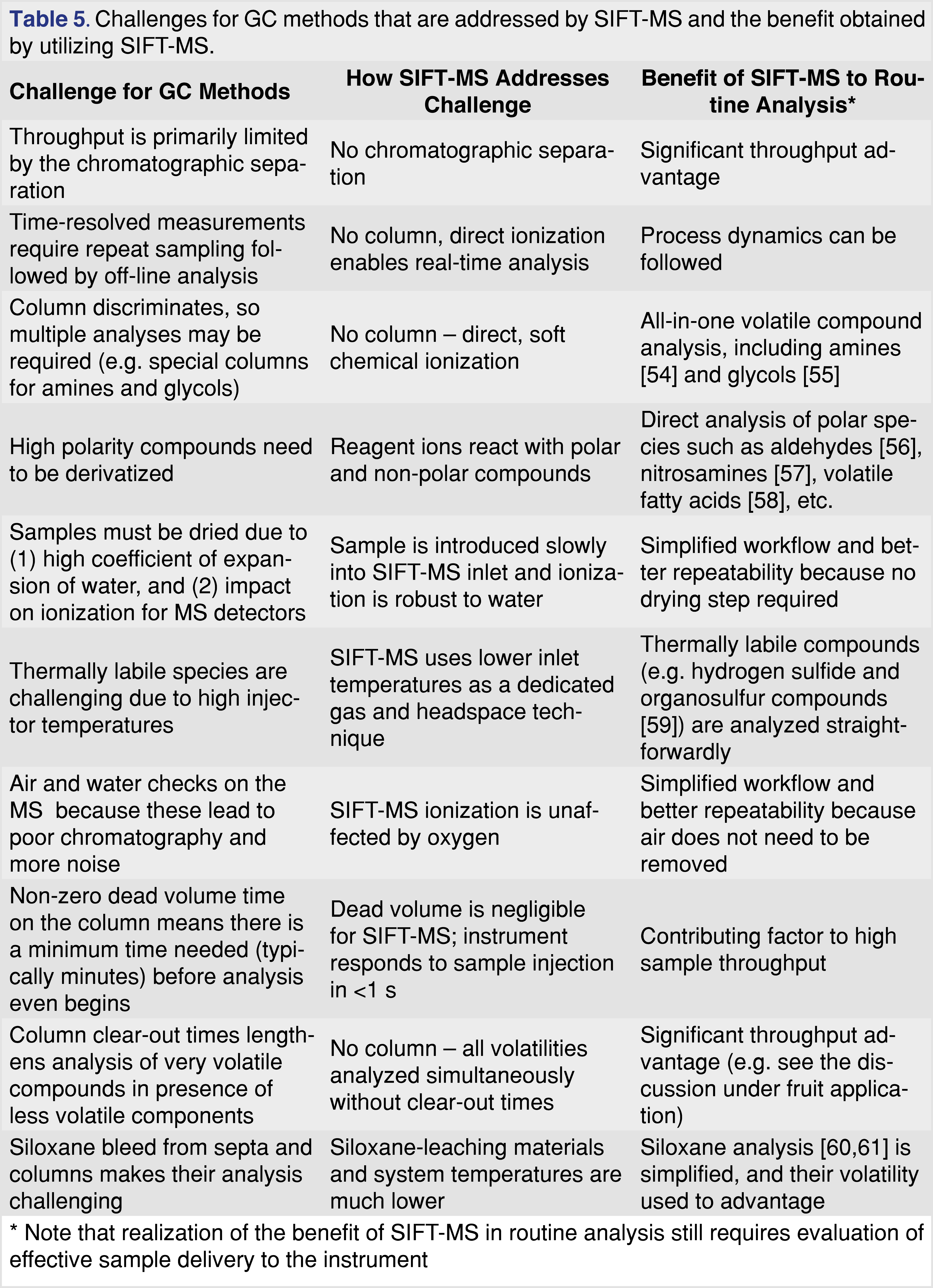
The SIFT-MS technique applies very soft chemical ionization that enables analysis of a wide range of volatile compounds in a single analysis with high selectivity, due to the diverse ionization mechanisms provided by the reagent ions [53]. This means that some of the common limitations of GC methods for gas and headspace analysis are addressed by implementing chromatography-free SIFT-MS analysis, as summarized in Table 5. Additionally, the speed with which static headspace analysis can be conducted with SIFT-MS [31] means that it can also be applied in support of method development for the other techniques for which headspace parameter optimization is ordinarily a protracted procedure and may be ignored.
In this section, two novel applications of SIFT-MS are briefly described that illustrate some of these benefits: (1) continuous headspace analysis of formaldehyde from polyoxymethylene polymer (POM) pellets, and (2) analysis of ethylene in fruit.
7.1 Case Study 1: Continuous Headspace Analysis (CHA)
CHA is a novel technique made possible by direct headspace analysis, such as that provided by SIFT-MS [62]. It enables emissions of volatile compounds to be probed in a dynamic, controlled manner, which is very challenging for conventional chromatographic methods. CHA differs from dynamic headspace analysis (DHA) in that real-time measurement of volatile emissions in the headspace is made throughout the process, whereas in DHA emissions are continuously trapped on a sorbent, then later thermally desorbed to give a single time-averaged measurement for the sample.
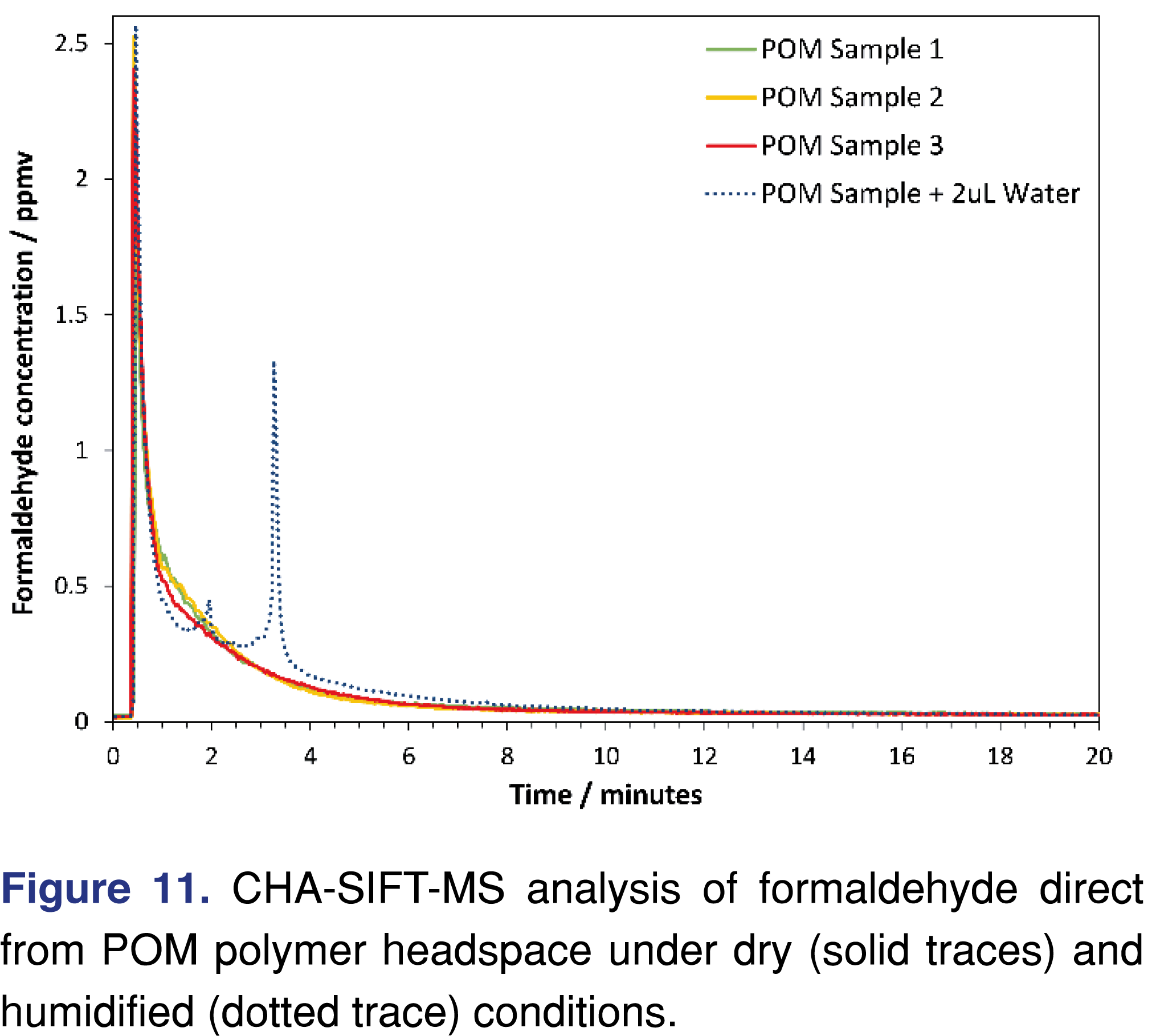
CHA is straightforward when using a SIFT-MS instrument integrated with a GERSTEL MPS autosampler equipped with a GERSTEL purge tool. Figure 11 shows replicate analyses of 22-mg samples of polyoxymethylene (POM) polymer pellets in standard 20-mL sample vials incubated at 60°C for 20 minutes. Data points have 2.7-s time resolution. Under dry purge conditions (zero air), formaldehyde concentrations demonstrate the expected decay. However, prior addition of water (2 µL) to the sample results in new structure (dotted curve in Figure 11). The area under each curve is very similar, indicating that the same amount of formaldehyde is being released from each sample. Addition of water hydrates the emitted formaldehyde, which then condenses on the vial walls for a short period before being released in a burst, producing the observed (and repeatable) spike in concentration at 3.25 minutes.
Similar principles apply to direct measurement of formaldehyde during thermal extraction of POM [63] and more general applications of SIFT-MS to formaldehyde analysis [19,37]. In summary, formaldehyde can be analyzed directly from just a few sccm in real-time and at high sensitivity using SIFT-MS, substantially simplifying sampling and analysis workflows.
7.2 Case Study 2: Rapid, Simple Analysis of Ethylene in Fruit
During ripening, fruits emit a diverse range of low molecular weight compounds arising from various hormonal and metabolic processes. The relative abundances of these volatiles change over time. Ethylene – because it promotes ripening – is usually of particular importance [64]. Conventional GC methodologies are usually applied, but this is challenging for ethylene and the solvent-like compounds emitted from fruits [65]. Direct analysis using SIFT-MS enables poorly retained compounds, such as ethylene, to be analyzed synchronously with the more retained volatiles in a GC analysis, eliminating the extended run time necessary in GC to clear the column of the less volatile, non-target compounds.
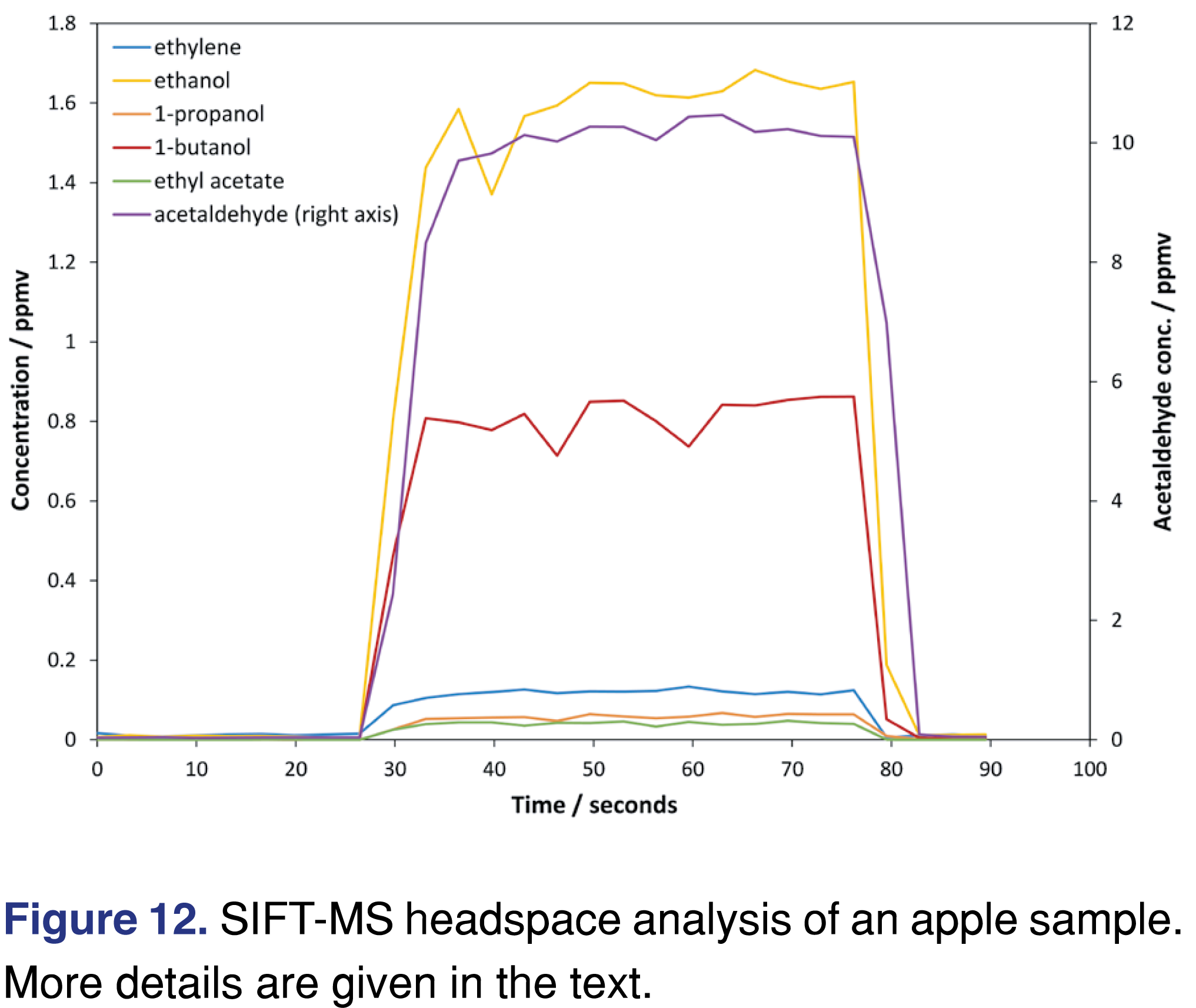
Application of SIFT-MS utilized 2-g apple samples in 20-mL autosampler vials, and an incubation temperature of 60 °C for 15 minutes [66]. Efficient scheduling of samples for analysis was achieved using commercially available software (GERSTEL Maestro; GERSTEL GmbH, Mülheim an der Ruhr, Germany). Figure 12 shows SIFT-MS data obtained for an apple sample, with all compound traces measured during the same injection. The background is visible at the start and end, and the rapid rise in headspace to a steady value occurs as the headspace sample is injected steadily into the SIFT-MS instrument’s inlet from the autosampler syringe. Comparing the SIFT-MS results with those in the GC/MS application data sheet [65] the relative levels are the same for both techniques. However, the analysis time is very different: compared to GC/MS, each SIFT-MS data point represents acquisition of results equivalent to an entire chromatogram in just a matter of seconds. Because SIFT-MS limits of quantitation improve with increased acquisition time [34], sample averaging over a total analysis time of several tens of seconds is employed. The concentration of each compound is derived from the mean value across the headspace injection.
SIFT-MS provides simple and highly sensitive and selective analysis of a poorly retained volatiles from fruits, with no delays due to late elution of non-targeted, less volatile compounds. Hence SIFT-MS addresses the rate limiting step in the conventional analysis: the chromatographic separation. The SIFT-MS method can analyze over six times more samples than the GC/MS method in a 24-hour period
8.0 Conclusion
Chromatography-based test methods have been the gold standard and workhorse for VOC analysis in routine testing laboratories and CROs for many years. However, they are not without their challenges – especially in terms of sample throughput, sample preparation (especially for polar compounds or wet samples), and discrimination introduced by the column or detector. Adoption of SIFT-MS has the potential to address these challenges through direct, soft CI of the sample gas or headspace by the reagent ions, while eliminating chromatographic separation. High specificity in real-time is achieved uniquely in SIFT-MS: multiple rapidly switchable chemical ionization agents (reagent ions) are utilized that often have different ionization mechanisms with different classes of VOCs. Throughput increases for room temperature analyses are up to 25-fold, incubated headspace analysis from 2 to 10-fold, and for the method or standard additions and multiple headspace extraction are from 2 to 6-fold.
This article has demonstrated how SIFT-MS fits comfortably in the routine testing laboratory and CRO, because routine analysis techniques developed for the chromatographic methods are readily adapted to suit it. Calibration and method validation approaches are increasingly utilized with SIFT-MS. However, some of the routine analysis procedures – in particular, use of internal standards and the method of standard additions – have not been utilized significantly with SIFT-MS due to the nature of the instrument (e.g. stability leading to high precision) and the predominant analyses that have been conducted (e.g. headspace). However, when needed they are easily implemented.
SIFT-MS will not replace the chromatographic methods in the routine analysis laboratory; rather, it complements them. It offers potential for high-throughput, economic screening prior to regulatory analyses, plus readily tackles a variety of compounds that are very challenging for conventional methods – especially species of low molecular weight that are polar and/or thermally labile.
9.0. References
- United States Environmental Protection Agency. Method 18 – Measurement of Gaseous Organic Compound Emissions by Gas Chromatography (2019). https://www.epa.gov/sites/production/files/2019-06/documents/method_18_0.pdf
- United States Environmental Protection Agency. Method 524.2 Measurement of Purgeable Organic Compounds in Water by Capillary Column Gas Chromatography/Mass Spectroscopy, Revision 4.1 (1995). https://www.epa.gov/sites/production/files/2015-06/documents/epa-524.2.pdf
- United States Environmental Protection Agency. Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air: Method TO-15, (2nd edn.), U.S. Environmental Protection Agency, Cincinnati, OH, EPA 600/625/R-96-010b (1999).
- United States Environmental Protection Agency. Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air: Method TO-17, (2nd edn.), U.S. Environmental Protection Agency, Cincinnati, OH, EPA 600/625/R-96-010b (1999).
- United States National Institute of Occupational Safety and Health. Method 2002 – Amines, Aromatic. Issue 2 (1994).
- [NIOSH2007] United States National Institute of Occupational Safety and Health. Method 2007 – Aminoethanol Compounds I. Issue 2 (1994).
- International Standards Organization. ISO-16000-6. Indoor Air – Part 6: Determination of Volatile Organic Compounds in Indoor and Test Chamber Air by Active Sampling on Tenax TA® Sorbent, Thermal Desorption and Gas Chromatography Using MS or MS-FID. Geneva (2011).
- International Standards Organization. ISO-12219-2. Interior Air of Road Vehicles – Part 2: Screening Method for the Determination of the Emissions of Volatile Organic Compounds from Vehicle Interior Parts and Materials – Bag Method. Geneva (2012).
- United States Pharmacopeia. ⟨476⟩ Residual Solvents.
- Donato P, Arena P, Mondello L. Theoretical and practical aspects of LC-MS analysis, in: Hyphenations of Capillary Chromatography with Mass Spectrometry, P.Q. Tranchida, L. Mondello (Eds.), Elsevier, Amsterdam, Netherlands, 297-317 (2020).
https://doi.org/10.1016/B978-0-12-809638-3.00008-9 - U.S. Environmental Protection Agency. Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air: Compendium Method TO-11A (2nd edn.), U.S. Environmental Protection Agency, Cincinnati, OH, EPA 600/625/R-96/010b (1999).
- Linforth RST, Taylor AJ. Apparatus and methods for the analysis of trace constituents in gases. European Patent. EP. 0819937A2 (1998).
- Lindinger W, Hirber J, Paretzke H. An ion/molecule‐reaction mass spectrometer used for online trace gas‐analysis. Int J Mass Spectrom Ion Processes 129, 79-88 (1993).
https://doi.org/10.1016/0168-1176(93)87031-M - Lagg A, Taucher J, Hansel A, Lindinger W. Applications of proton transfer reactions to gas analysis. Int J Mass Spectrom Ion Processes 134, 55-66 (1994).
https://doi.org/10.1016/0168-1176(94)03965-8 - Spanel P, Smith D. Selected ion flow tube: a technique for quantitative trace gas analysis of air and breath. Med Biol Eng Comput 34, 409-419 (1996).
https://doi.org/10.1007/BF02523843 - Smith D, Spanel P. Selected ion flow tube mass spectrometry (SIFT‐MS) for on‐line trace gas analysis. Mass Spectrom Rev 24, 661-700 (2005).
https://doi.org/10.1002/mas.20033 - McEwan MJ, Direct analysis mass spectrometry, in: Ion Molecule Attachment Reactions: Mass Spectrometry, T. Fujii (Ed.), Springer, New York, USA, 263-317 (2015).
https://doi.org/10.1007/978-1-4899-7588-1_8 - Langford VS, Padayachee D, McEwan MJ, Barringer SA. Comprehensive odorant analysis for on-line applications using selected ion flow tube mass spectrometry (SIFT-MS). Flav Frag J 34(6), 393-410 (2019).
https://doi.org/10.1002/ffj.3516 - Langford VS, Perkins MJ, Milligan DB, Prince BJ, McEwan MJ. High-speed formaldehyde analysis for the process-line and laboratory: SIFT-MS. International Society of Automation (ISA) 62nd Analysis Division Symposium 2017, Pasadena, CA, USA.
- Hera D, Langford VS, McEwan MJ, McKellar TI, Milligan DB. Negative reagent ions for real time detection using SIFT-MS. Environments 4, 16 (2017).
https://doi.org/10.3390/environments4010016 - Langford VS, McEwan MJ, Askey M, Barnes HA, Olerenshaw JG. Comprehensive instrumental odor analysis using SIFT-MS: a case study. Environments 5(4), 43 (2018).
https://doi.org/10.3390/environments5040043 - Langford VS, Billiau C, McEwan MJ. Evaluation of the efficacy of SIFT-MS for speciation of wastewater treatment plant odors in parallel with human sensory analysis. Environments 7, 90 (2020).
https://doi.org/10.3390/environments7100090 - Langford VS, Du Bruyn C, Padayachee D. An evaluation of selected ion flow tube mass spectrometry for rapid instrumental determination of paper type, origin and sensory attributes. Packag Technol Sci 34, 245-260 (2021).
https://doi.org/10.1002/pts.2555 - Becker H, Bacquart T, Perkins M, Moore N, Ihonen J, Hinds G, Smith G. Operando characterization of the impact of carbon monoxide on PEMFC performance using isotopic labelling and gas analysis. J Power Sources Adv 6, 100036 (2020).
https://doi.org/10.1016/j.powera.2020.100036 - Lobaccaro P, Mandal L, Motapothula MR, Sherburne M, Martin J, Venkatesan T, Ager JW. Initial application of selected‐ion flow‐tube mass spectrometry to real‐time product detection in electrochemical CO2 reduction. Energy Technol 6, 110-121 (2018).
https://doi.org/10.1002/ente.201700628 - La Nasa J, Mattonai M, Modugno F, Degano I, Ribechini E. Comics’ VOC-abulary: study of the ageing of comic books in archival bags through VOCs profiling. Polymer Degrad Stab 161, 39-49 (2019).
https://doi.org/10.1016/j.polymdegradstab.2019.01.001 - La Nasa J, Modugno F, Colombini MP, Degano I. Validation study of selected ion flow tube-mass spectrometry (sift-ms) in heritage science: characterization of natural and synthetic paint varnishes by portable mass spectrometry. J Am Soc Mass Spectrom 30, 2250-2258 (2019).
https://doi.org/10.1007/s13361-019-02305-4 - La Nasa J, Lomonaco T, Manco E, Ceccarini A, Fuoco R, Corti A, Modugno F, Castelvetro V, Degano I. Plastic breeze: volatile organic compounds (VOCs) emitted by degrading macro- and microplastics analyzed by selected ion flow-tube mass spectrometry. Chemosphere 128612 (2020).
https://doi.org/10.1016/j.chemosphere.2020.128612 - Perkins MJ, Padayachee D, Langford VS, McEwan MJ. High throughput residual solvent and residual monomer analysis using selected ion flow tube mass spectrometry. Chromatogr Today 10(3), 2‐5 (2017).
- Perkins MJ, Langford VS, McEwan MJ. High‐throughput analysis of volatile compounds in air, water and soil using SIFT‐MS. Curr Trends Mass Spectrom 37, 24-29 (2018).
- Perkins MJ, Langford VS. V”Standard Validation Protocol for Selected Ion Flow Tube Mass Spectrometry Methods Applied to Direct Headspace Analysis of Aqueous Volatile Organic Compounds”. Submitted.
- Spanel P, Smith D. SIFT studies of the reactions of H3O+, NO+ and O2+ with several aromatic and aliphatic hydrocarbons. Int J Mass Spectrom 181, 1-10 (1998).
https://doi.org/10.1016/S1387-3806(98)14114-3 - Ridgway K. Method validation of volatile organic compounds (VOCs) in water using the Anatune robotic VOC analyser”. Anatune Application Note AS243; 2021. Available at www.anatune.co.uk.
- Milligan DB, Francis GJ, Prince BJ, McEwan MJ. Demonstration of selected ion flow tube MS detection in the parts per trillion range. Anal Chem 79, 2537-2540 (2007).
https://doi.org/10.1021/ac0622678 - Hoffmann A, MacNamara K. Large volume injection with solvent venting-application to trace detection of analytes in water. GERSTEL Application Note AN/2012/15-2, (2012).
- International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH). Validation of Analytical Procedures: Text and Methodology Q2(R1) (1996).
- Wicks J, Perkins MJ, Langford VS. Validation of a high-throughput SIFT-MS method for analysis of formaldehyde leachate. Syft Application Note 55 (2019). Available at www.syft.com.
- U.S. Environmental Protection Agency. Method 5021A – Volatile organic compounds in various sample matrices using equilibrium headspace analysis, Revision 2 (2014). Available at https://www.epa.gov/sites/production/files/2015-12/documents/5021a.pdf
- U.S. Environmental Protection Agency. Method 5035 – Closed-system purge-and-trap and extraction for volatile organics in soil and waste samples, Revision 0 (1996). Available at https://www.epa.gov/sites/production/files/2015-12/documents/5035.pdf
- Carrier, D. Davis, R. Automated approach for determination of BTEX in Soil. Anatune Application Note AS151 (2016). Available at www.anatune.co.uk.
- Perkins MJ. Methanolic extraction of soils by automated selected ion flow tube mass spectrometry (SIFT-MS). Anatune Application Note AS244 (2021). Available at www.anatune.co.uk.
- Spanel P, Smith D. SIFT studies of the reactions of H3O+, NO+ and O2+ with a series of alcohols. Int J Mass Spectrom Ion Proc 167/168, 375-388 (1997).
https://doi.org/10.1016/S0168-1176(97)00085-2 - Biba E, Perkins MJ, Langford VS. High-throughput residual solvent analysis using selected ion flow tube mass spectrometry (SIFT-MS). Manuscript in preparation.
- Prince BJ, Milligan DB, McEwan MJ. Application of selected ion flow tube mass spectrometry to real‐time atmospheric monitoring. Rapid Commun Mass Spectrom 24, 1763‐1769 (2010).
https://onlinelibrary.wiley.com/doi/10.1002/rcm.4574 - Syft Technologies Limited. Automated validation of Voice-series instruments. Syft Technologies Technical Note TNE-016 (2012). Available at www.syft.com.
- Perkins MJ, Langford VS. Rapid analysis of BTEX in water using automated SIFT-MS. Syft Technologies Application Note APN-045 (2018). Available at www.syft.com.
- Bell KJ, Ma J, Padayachee D, Langford VS. Rapid analysis of BTEX using thermal desorption-SIFT-MS. Syft Technologies Application Note APN-048 (2019). Available at www.syft.com.
- Hastie C, Thompson A, Perkins M, Langford V, Eddleston M, Homer, N. Selected ion flow tube-mass spectrometry (SIFT-MS) as an alternative to gas chromatography/mass spectrometry (GC/MS) for the analysis of cyclohexanone and cyclohexanol in plasma. Manuscript in preparation.
- Perkins MJ. Unpublished data (2019).
- Zhu Y, Li G, Duan Y, Chen S, Zhang C, Li Y. Application of the standard addition method for the determination of acrylamide in heat-processed starchy foods by gas chromatography with electron capture detector. Food Chem 109(4), 899-908 (2008).
https://doi.org/10.1016/j.foodchem.2008.01.020 - Frenich AG, Vidal JLM, Moreno JLF, Romero-González R. Compensation for matrix effects in gas chromatography-tandem mass spectrometry using a single point standard addition. J Chrom A 1216(23), 4798-4808 (2009).
https://doi.org/10.1016/j.chroma.2009.04.018 - Król S, Zabiega B, Namie J. Determination of polybrominated diphenyl ethers in house dust using standard addition method and gas chromatography with electron capture and mass spectrometric detection. J Chrom A 1249, 201-214 (2012).
https://doi.org/10.1016/j.chroma.2012.06.001 - Smith D, McEwan MJ, Spanel P. Understanding gas phase ion chemistry is the key to reliable selected ion flow tube-mass spectrometry analyses. Anal Chem 92, 12750-12762 (2020).
https://doi.org/10.1021/acs.analchem.0c03050 - Spanel P, Smith D. Selected ion flow tube studies of the reactions of H3O+, NO+, and O2+ with several amines and some other nitrogen-containing molecules. Int J Mass Spectrom 176, 203-211 (1998).
https://doi.org/10.1016/S1387-3806(98)14031-9 - Spanel P, Wang T, Smith D. A selected ion flow tube, SIFT, study of the reactions of H3O+, NO+ and O2+ ions with a series of diols. Int J Mass Spectrom 218, 227-236 (2002).
https://doi.org/10.1016/S1387-3806(02)00724-8 - Spanel P, Ji Y, Smith D. SIFT studies of the reactions of H3O+, NO+ and O2+ with a series of aldehydes and ketones. Int J Mass Spectrom Ion Proc 165/166, 137-147 (1997).
https://doi.org/10.1016/S0168-1176(97)00166-3 - Langford VS, Gray JDC, Maclagan RGAR, Milligan DB, McEwan MJ. Real-time measurements of nitrosamines in air. Int J Mass Spectrom 377, 490-495 (2015).
https://doi.org/10.1016/j.ijms.2014.04.001 - Spanel P, Smith D. SIFT studies of the reactions of H3O+, NO+ and O2+ with a series of volatile carboxylic acids and esters. Int J Mass Spectrom Ion Proc 172, 25-37 (1998).
https://doi.org/10.1016/S0168-1176(97)00246-2 - Spanel P, Smith D. Selected ion flow tube studies of the reactions of H3O+, NO+, and O2+ with some organosulphur molecules. Int J Mass Spectrom 176, 167-176 (1998).
https://doi.org/10.1016/S1387-3806(98)14004-6 - Langford VS, Gray JD, Maclagan RG, McEwan MJ. Detection of siloxanes in landfill gas and biogas using SIFT-MS. Curr Anal Chem 9, 558-564 (2013).
https://doi.org/10.2174/15734110113099990020 - Langford VS. Gray JDC, McEwan MJ. Selected ion flow tube studies of several siloxanes. Rapid Commun Mass Spectrom 27, 700-706 (2013).
https://doi.org/10.1002/rcm.6496 - Perkins MJ, Langford VS. Continuous headspace analysis using SIFT-MS: a powerful probe of dynamic processes. Syft Application Note APN-028 (2018). Available at www.syft.com.
- Bell KJM, Ma J, Padayachee D, Langford VS, Perkins MJ. Time-resolved thermal extraction of residual formaldehyde using thermal desorption-SIFT-MS. Syft Application Note APN-049 (2019). Available at www.syft.com.
- Caprioli F, Quercia L, Ethylene detection methods in post-harvest technology: a review. Sens Act B Chem 203, 187-196 (2014).
https://doi.org/10.1016/j.snb.2014.06.109 - Shimadzu Corporation. Analysis of ethylene in food using GC/MS. Shimadzu Application Datasheet (2015).
- Perkins MJ, Langford VS. High-throughput analysis of volatiles from fruit using SIFT-MS. Syft Application Note APN-031 (2018). Available at www.syft.com.
All site content, except where otherwise noted, is licensed under a Creative Commons Attribution 4.0 License