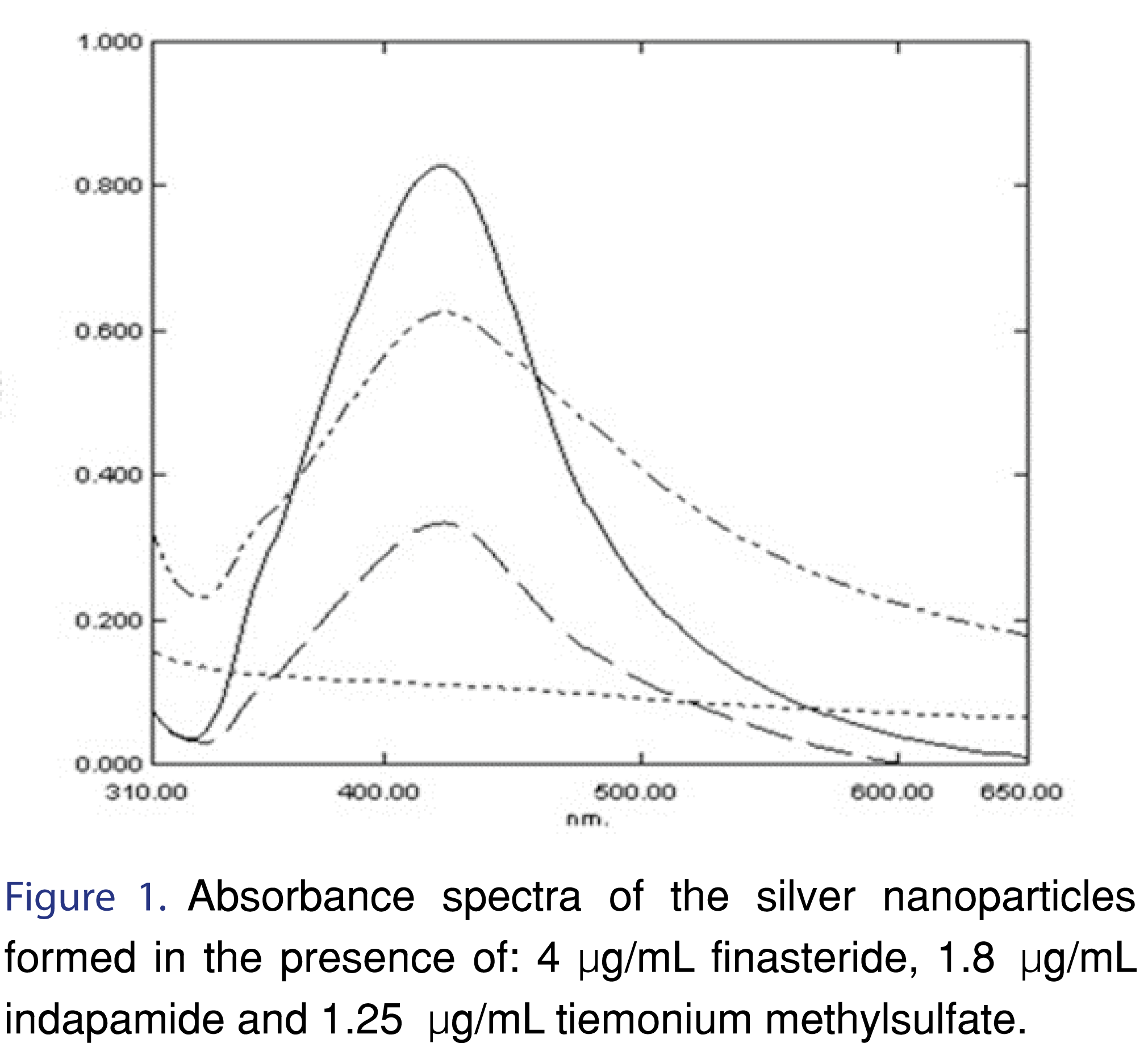
OPEN-ACCESS PEER-REVIEWED
RESEARCH ARTICLE
Magda Mohamed Ayad, Hisham Ezzat Abdellatef, Mervat Mohamed Hosny* ,Naglaa Abdel-Sattar Kabil
Analytical Chemistry Department, Faculty of Pharmacy, Zagazig University, Zagazig, Egypt 44519.
Reviews in Separation Sciences. Vol.2. No.1. pages 5-18 (2020).
Published 15 July 2020. https://doi.org/10.17145/rss.20.002 | (ISSN 2589-1677).
- Abstract
- Keywords
- 1.0 Introduction
- 2.0 Experimental
- 2.2 Materials and reagents
- 2.3. Pharmaceutical preparations
- 2.4. Standard solutions
- 2.5. General procedures
- 2.6 Assay of Pharmaceutical Preparations Assay
- 2.7 Procedures for Content Uniformity Testing
- 3.0 Results and discussion
- 3.1 Optimization of experimental variables
- Figures and Tables
- 3.2. Method validation
- 4.0 Conclusion
- 5.0 Acknowledgments
- 6.0 References
*Correspondence:
Hosny MM. . Analytical Chemistry Department, Faculty of Pharmacy, Zagazig University, Zagazig, Egypt 44519.
Editor: Dr. Inas Abdallah, Faculty of Pharmacy, University of Sadat City, Egypt.
Open-access and Copyright:
©2020 Hosny MM et al. This article is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Funding/Manuscript writing assistance:
The authors have financial support or funding to report and they declare that no writing assistance was utilized in the production of this article.
Competing interest:
The authors have declared that no competing interest exist.
Article history:
Received 19 July 2019, Revised 20 October 2019, Accepted 26 October 2019.
Abstract
A simple and sensitive method was developed for spectrophotometric determination of finasteride, indapamide and tiemonium methylsulfate in their pure form and in their pharmaceutical formulations. It was found that the studied drugs have the ability to reduce silver nitrate to silver nanoparticles (AgNPs) in the presence of sodium citrate as a stabilizing agent. Silver nanoparticles (AgNPs) produce a very intense surface plasmon resonance peak at 423 nm that allows the quantitative determination of the studied drugs. The calibration curves were linear with concentrations range of 0.50–5.00, 0.50-5.00 and 0.30-2.00 μg/mL for finasteride, indapamide and tiemonium methylsulfate, respectively. The proposed method was successfully applied to the determination of the studied drugs in their pharmaceutical formulations. Furthermore, content uniformity testing of the studied pharmaceutical tablets was also conducted.
Keywords
silver nanoparticles, finasteride, indapamide and tiemonium methyl sulphate.
1.0 Introduction
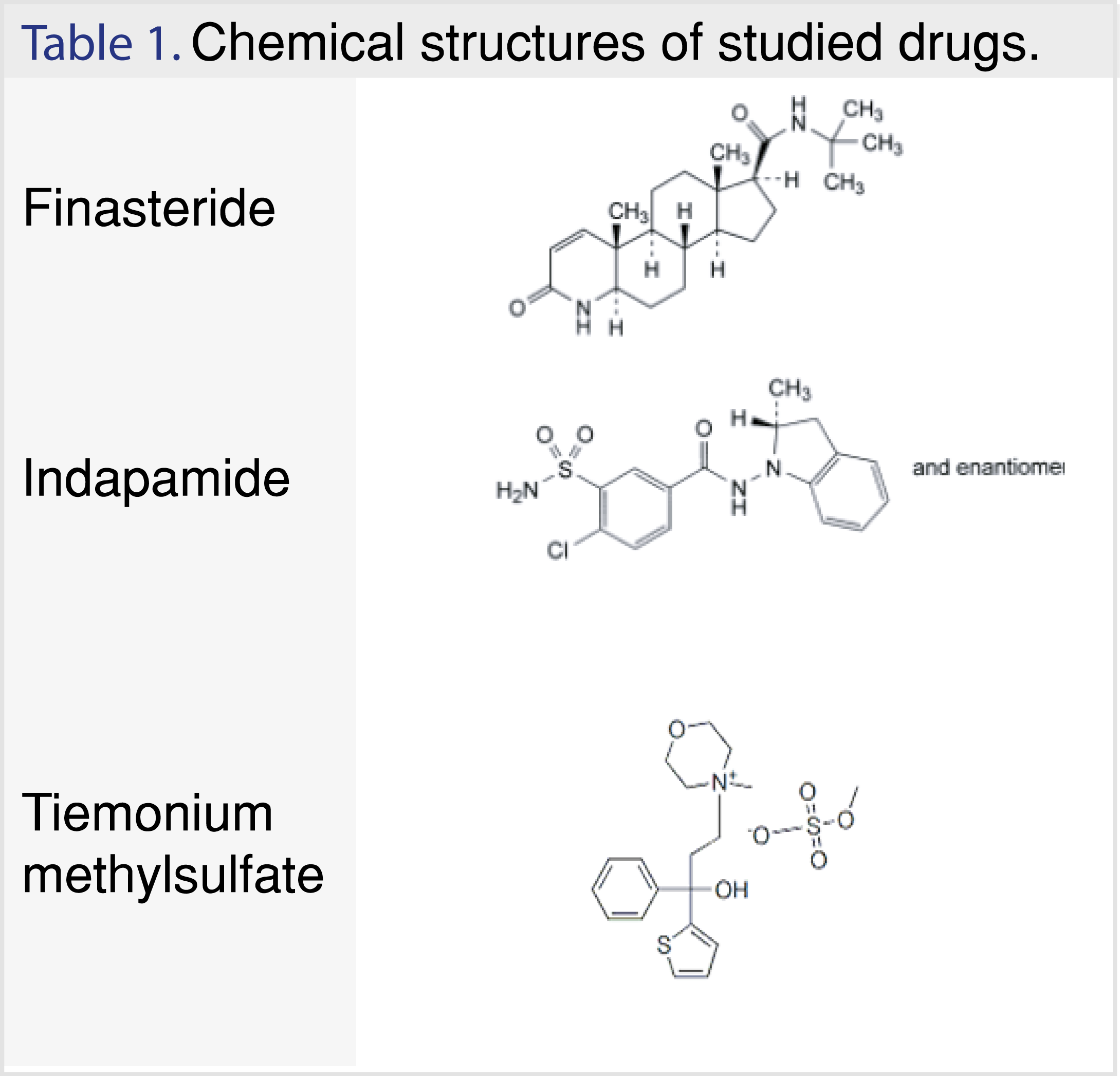
Finasteride is N-(1,1-Dimethylethyl)-3-oxo-4-aza-5a-androst-1-ene-17b-carboxamide (Table 1).
It is an azasteroid that inhibits the type-2 isoform of 5-reductase, the enzyme responsible for the conversion of testosterone to the more active dihydrotestosterone, and has therefore anti-androgenic properties. It is given daily orally in a dose of 5 mg in the management of benign prostatic hyperplasia to cause regression of the enlarged prostate and to improve symptoms; it may reduce the incidence of acute urinary retention and the need for surgery [1]. Finasteride is an official drug listed in British Pharmacopeia (BP) [2] and it can be determined using HPLC.
Research Article
Several methods have been reported for finasteride determination including spectrophotometry [3-7], HPLC [8-10], HPTLC [11], voltammetry [12] and polarography [13].
Indapamide is 4-Chloro-N-[(2RS)-2-methyl-2,3-dihydro-1H-indol-1-yl]-3-sulphamoylbenzamide (Table 1).
The drug exerts diuretic actions similar to thiazide diuretics, despite lacking a thiazide moiety in the drug. It is used for treating hypertension and oedema associated with heart failure [1]. Indapamide is an official drug listed in BP [2] and it can also be determined using HPLC. Different techniques were reported for its determination including spectrophotometry [14-18], chromatographic methods [19-21] and voltammetry [22].
Tiemonium methylsulfate is 4-[3-Hydroxy-3-phenyl-3-(2-thienyl)propyl]-4-methylmorpho-linium methylsulfate (Table 1).
It is used for the relief of visceral spasms [1]. Tiemonium methylsulfate is not listed in any of the pharmacopoeias. Thus a thorough literature search was performed on this drug. Most of the reported methods used spectroscopic techniques, this due to their simplicity [23-27]. Chromatographic methods [28,29] and electrochemical methods [30] were also used for determination. Recently silver nanoparticles have reported having wide applications in various areas of chemistry due to their unique optical properties [31-34]. In this work, we report a simple and sensitive method for the determination of finasteride, indapamide and tiemonium methylsulfate. This assay is based on the spectrophotometric determination of silver nanoparticles at 423 nm, which were formed due to the reduction of silver nitrate by the aforementioned drugs in the presence of sodium citrate.
2.0 Experimental
2.1. Instrumentation
A single cell holder JENWAY 6715 UV/Visible spectrophotometer (UK) equipped with 10 mm matched quartz cells was employed for all absorbance measurements. Furthermore, a vortex mixer model VELP® (Scientifica RX3) and a centrifuge model Hettich Zentrifugen Universal 320/320 R (Germany) were used.
2.2 Materials and reagents
Finasteride was obtained from SIGMA pharmaceutical industries, Egypt. Its purity was found to be 99.92% according to the comparison method [6]. Indapamide was obtained from Pharco pharmaceuticals, Alexandria, Egypt. Its purity was found to be 99.95% according to the comparison method [17]. Tiemonium methylsulfate was obtained from Adwia Pharmaceutical Industries Co.(Cairo, Egypt). Its purity was found to be 99.9% as reported by the company. Its purity was found to be 100.40% according to the comparison method [23]. Silver nitrate (AgNO3) was obtained from Morgan Speciality Chemicals Company and its purity was found to be 99.5% as reported (Batch No 572070216). Sodium citrate was obtained from Fischer Chemical (Fischer scientific UK limited, UK) and Sodium hydroxide was obtained from Alpha Chemicals and was for laboratory use. Its purity was found to be 98% as reported by the company.
2.3. Pharmaceutical preparations
Prostride® capsules containing 5 mg finasteride per capsule (obtained from Adwia Pharmaceutical Industries Co., Cairo, Egypt) Batch No. 1603221.
Hypotense® tablets containing 2.5 mg indapamide per tablet (obtained from the Arab Drug Company, Cairo, Egypt) Batch No. 210189.
Normaten® tablets containing 2.5 mg indapamide and 50 mg captopril per tablet (obtained from Tenth of Ramadan For Pharmaceutical Industries & Diagnostic Reagent (rameda), 6th of October city, Egypt) Batch No. 170439.
Visceralgine® tablets containing 50 mg tiemonium methylsulfate per tablet (obtained from Sedico Pharmaceutical Company, Giza, Egypt) Batch No. 0916299.
Viscera® ampoules, containing 5 mg timonium methylsulfate per 2 mL (Sedico Pharmaceutical Company, Giza, Egypt), Batch No. 1216287/A.
2.4. Standard solutions
2.4.1 Standard Stock Solution
A standard stock solution containing 1mg/mL of each drug was prepared separately in ethanol, methanol, and water for finasteride, indapamide and tiemonium methylsulfate, respectively.
2.4.2 Working standard solutions
The standard stock solution of each drug was diluted separately, by the same solvent of each drug, to obtain a concentration of 10 μg/mL.
2.5. General procedures
In a 5 mL volumetric flask appropriate amounts of silver nitrate, sodium citrate, drugs (finasteride, indapamide, tiemonium methylsulfate), and sodium hydroxide, only for finasteride, were added to make up the volume with distilled water. Each solution was heated in water at a suitable temperature for appropriate times. Absorbance was measured at the suitable wavelength against reagent blank treated similarly (Table 2).
2.6 Assay of Pharmaceutical Preparations Assay
2.6.1 Tablets Assays
2.6.1.1 Assay of Hypotense® and Normaten® Tablets
Ten tablets were weighed and pulverized. Then, the powder accounting for 10 mg of drugs was transferred into a 10 mL volumetric flask. The powder was dissolved using 1 mL of 0.05M HCl and diluted to mark using methanol. Solutions were filtered and neutralized with 1 mL of 0.05 M NaOH and diluted to 10 μg/mL. Aliquots from this solution were used for subsequent experiments.
2.6.1.2 Assay of Visceralgine® tablets
Ten tablets were weighed, pulverized into a fine powder, in a 10 mL volumetric flask specific quantity of powdered tablets equivalent to 10.0 mg pure drugs were dissolved, diluted to mark using methanol and sonicated for 30 minutes. Solutions were filtered and then further diluted to 10 μg/mL. Aliquots from this solution were used for subsequent experiments.
2.6.2 Assay of Prostride® capsules
The contents of ten capsules were emptied in a 10 mL volumetric flask and accurately weighed the amount of finasteride equivalent to 10 mg was dissolved and diluted to the mark using ethanol. The drug solution was filtered and further diluted to 10μμg/mL and aliquots from this solution were used for subsequent experiments.
2.6.3 Assay of Viscera® ampoule
Specific volumes of ampule solutions equivalent to 10.0 mg pure drug were placed in a 100.0 mL volumetric flask, diluted to 100.0 mL with 50% methanol (v/v). The drug solution was then diluted to 10μμg/mL and aliquots from this solution were used for subsequent experiments.
2.7 Procedures for Content Uniformity Testing
Each of the ten tablets of Hypotense®, and capsules of Prosteride® were weighted accurately. Each tablet or capsule was considered a sample and analyzed as previously mentioned (section 2.6). The content of the drug present in the tablets was calculated as a percent of the label claim for each tablet or capsule, respectively. The percent drug content of the label claim was assessed to see if it complied with the acceptance criteria.
2.7.1 Procedures for application of tiemonium methyl sulfate into plasma
In a centrifuge tube were 0.5 mL of 20 μg/mL tiemonium methylsulfate mixed with 0.5 mL of plasma using vortexing. Thereafter, 4.0 mL of acetonitrile were added to the tube, mixed well for 1 minute and centrifuged for 30 min at 5000 rpm. The supernatant was passed through a cellulose acetate syringe filter. In 5 mL volumetric flask, 0.15 mL of the supernatant was added to different concentrations of pure drug. The procedures were completed as previously mentioned under general procedures (section 2.5).
3.0 Results and discussion
In recent years, silver nanoparticles have been reported in many applications. They have gained much interest in chemical analysis due to their high extinction coefficient and cost-effectiveness of the analysis. In an alkaline medium; silver nitrate was reduced by the studied drugs into silver nanoparticles which were stabilized by a sodium citrate solution (Figure 1). Silver nanoparticles exhibit a well-known absorption band at 423 nm that has successfully been utilized in the determination of the cited drugs Figure 1.
3.1 Optimization of experimental variables
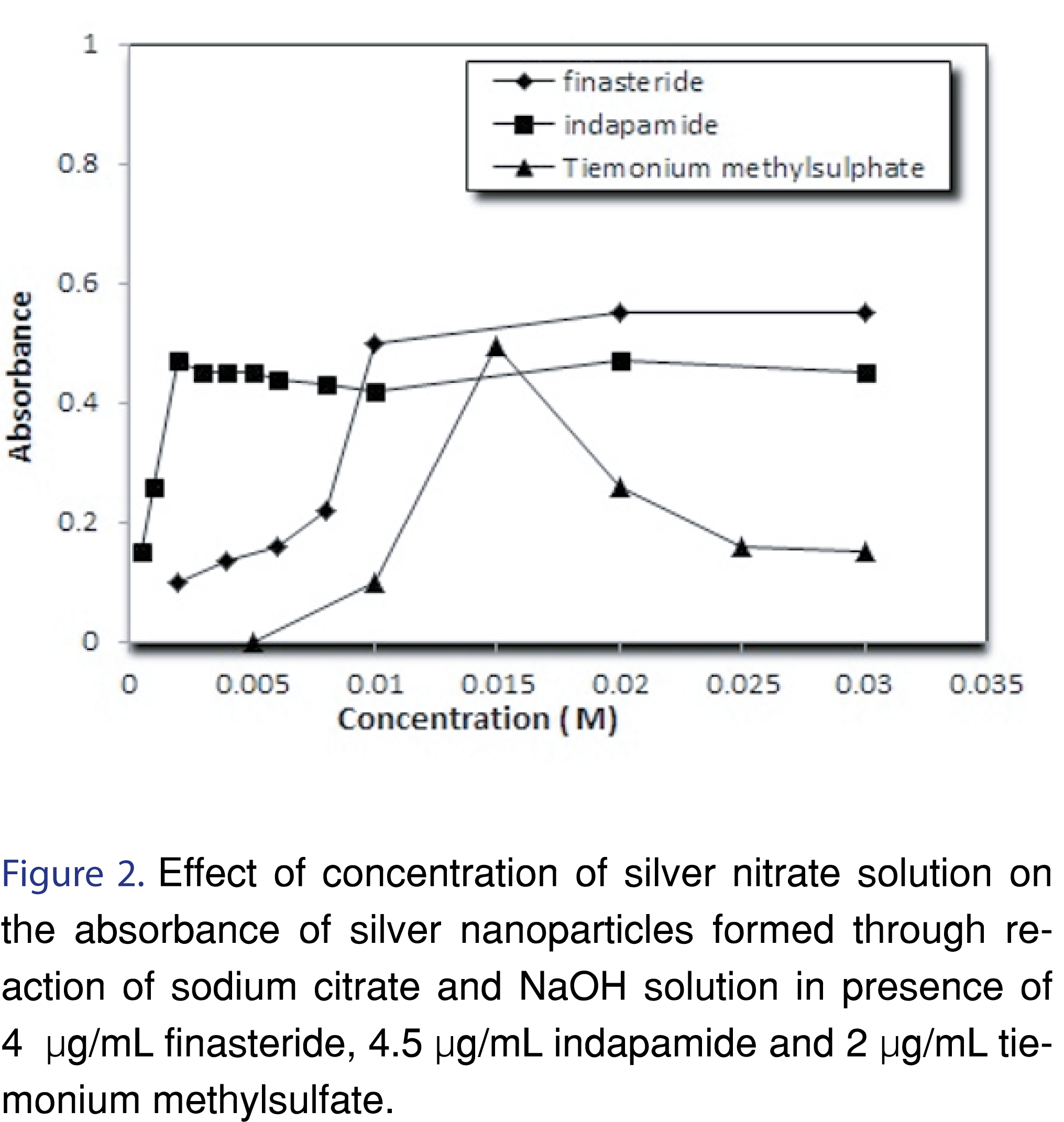
3.1.1 Effect of silver nitrate
In order to find the optimum concentration of silver nitrate, different concentrations from 0.5 to 30 mM were examined as shown in Figures 2, 3 and Table 2.
3.1.2 Effect of Stabilizer type and concentration
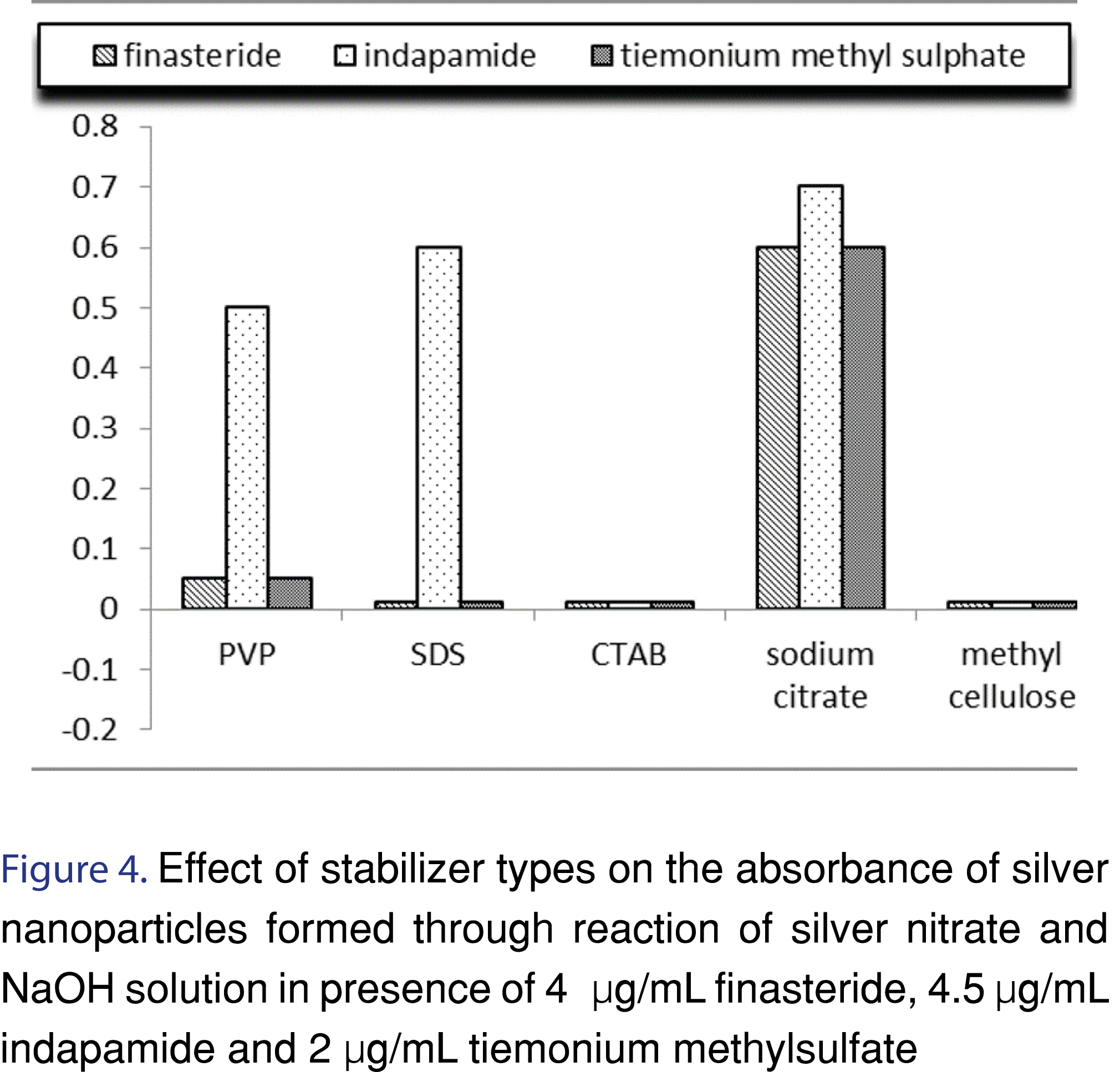
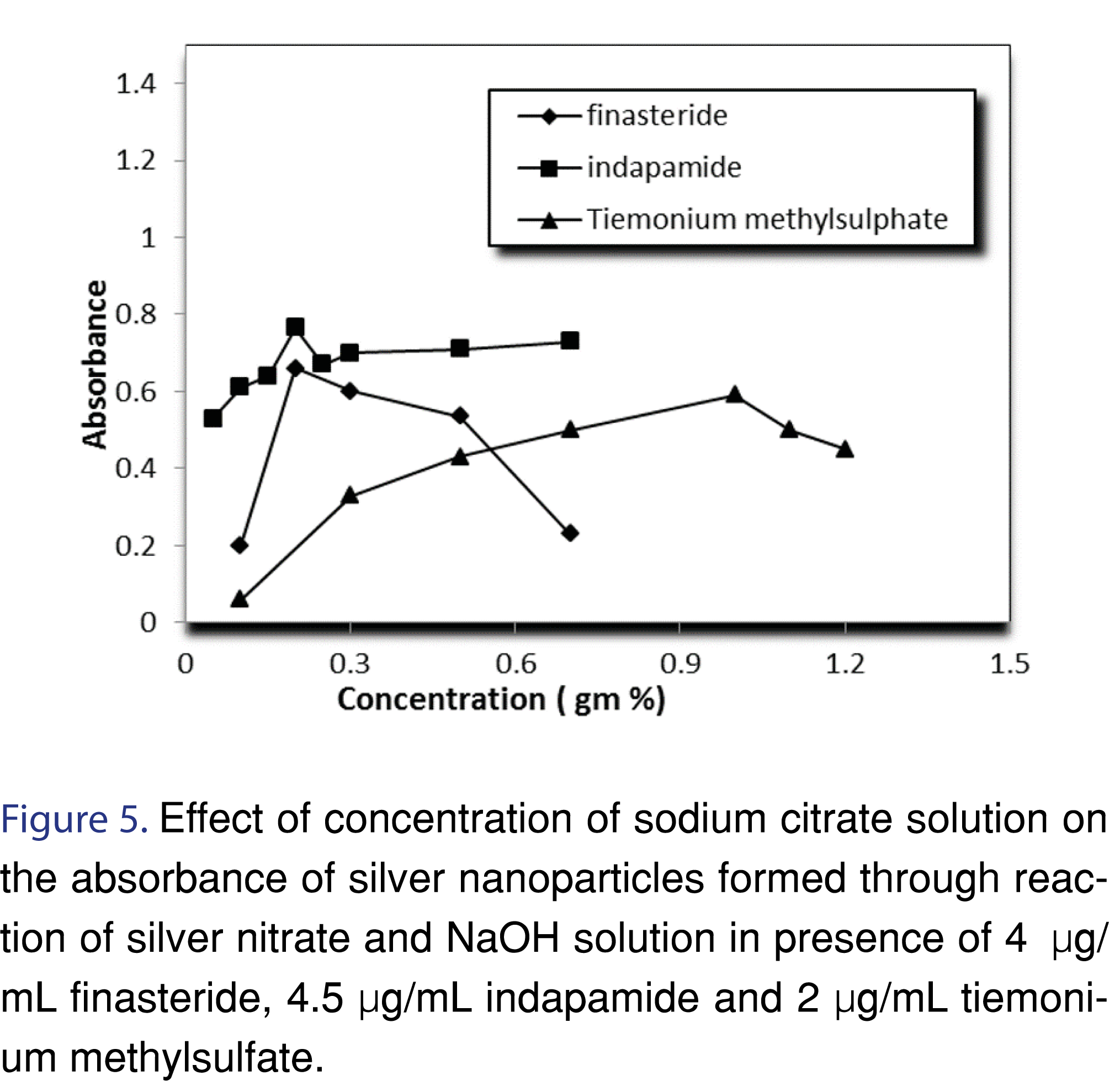
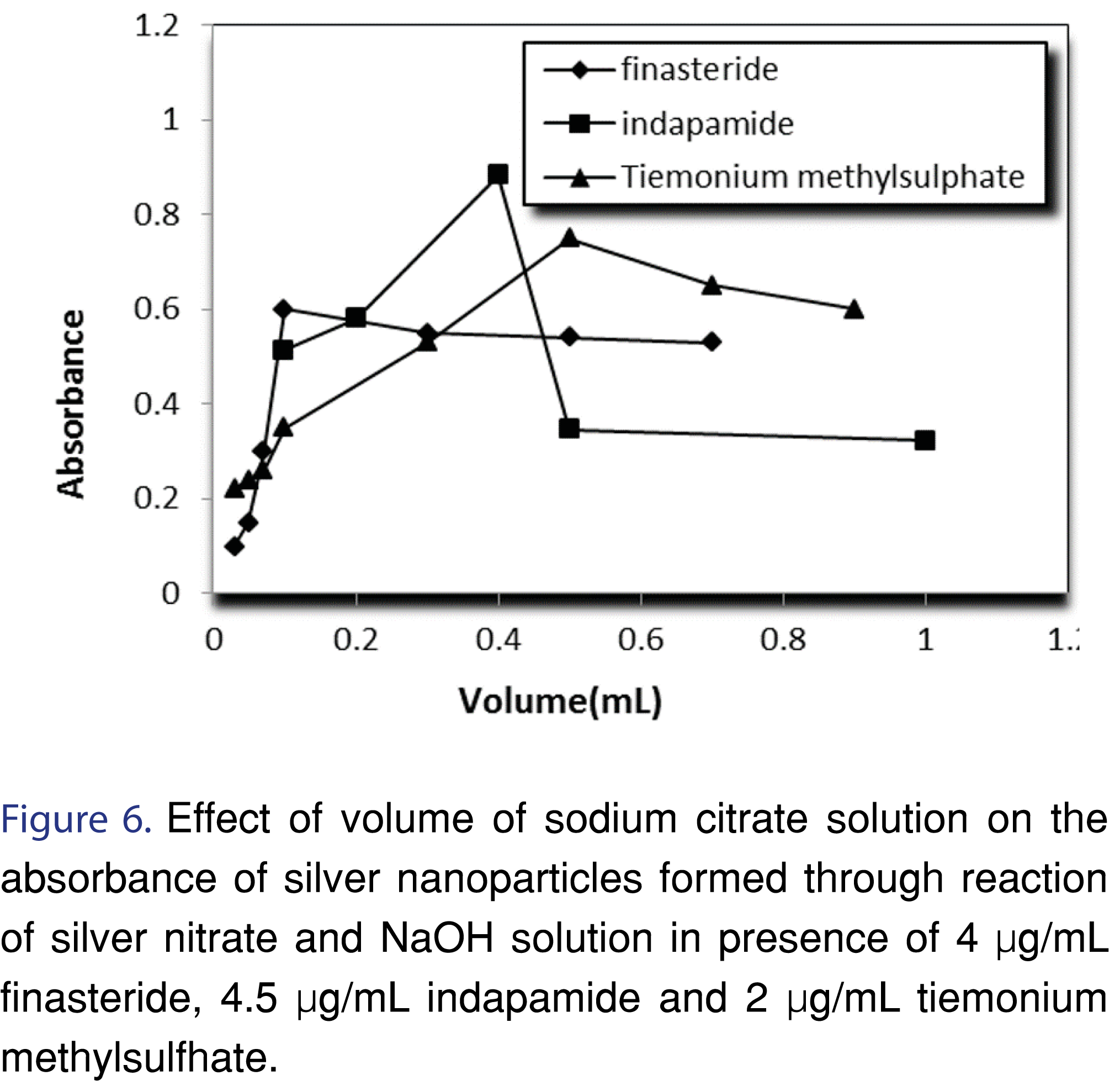
Stabilization of silver nanoparticles is very important to prevent their aggregation. Nanoparticles stabilization is achieved by two mechanisms: electrostatic- and steric stabilization. Electrostatic stabilization is caused by the repulsion between particles, (e.g. sodium citrate) while steric stabilization is achieved by surrounding the metal center by surfactants or polymers (e.g. PVP). In this study polyvinyl pyrrolidone (PVP), sodium citrate, sodium dodecyl sulphate (SDS), cetyl trimethyl ammonium bromide (CTAB) and methyl cellulose were tried as a stabilizing agent. Sodium citrate was selected as the best stabilizer for the prevention of AgNPs agglomeration Figure 4-6 and Table 2.
Figures and Tables
[Click to enlarge]
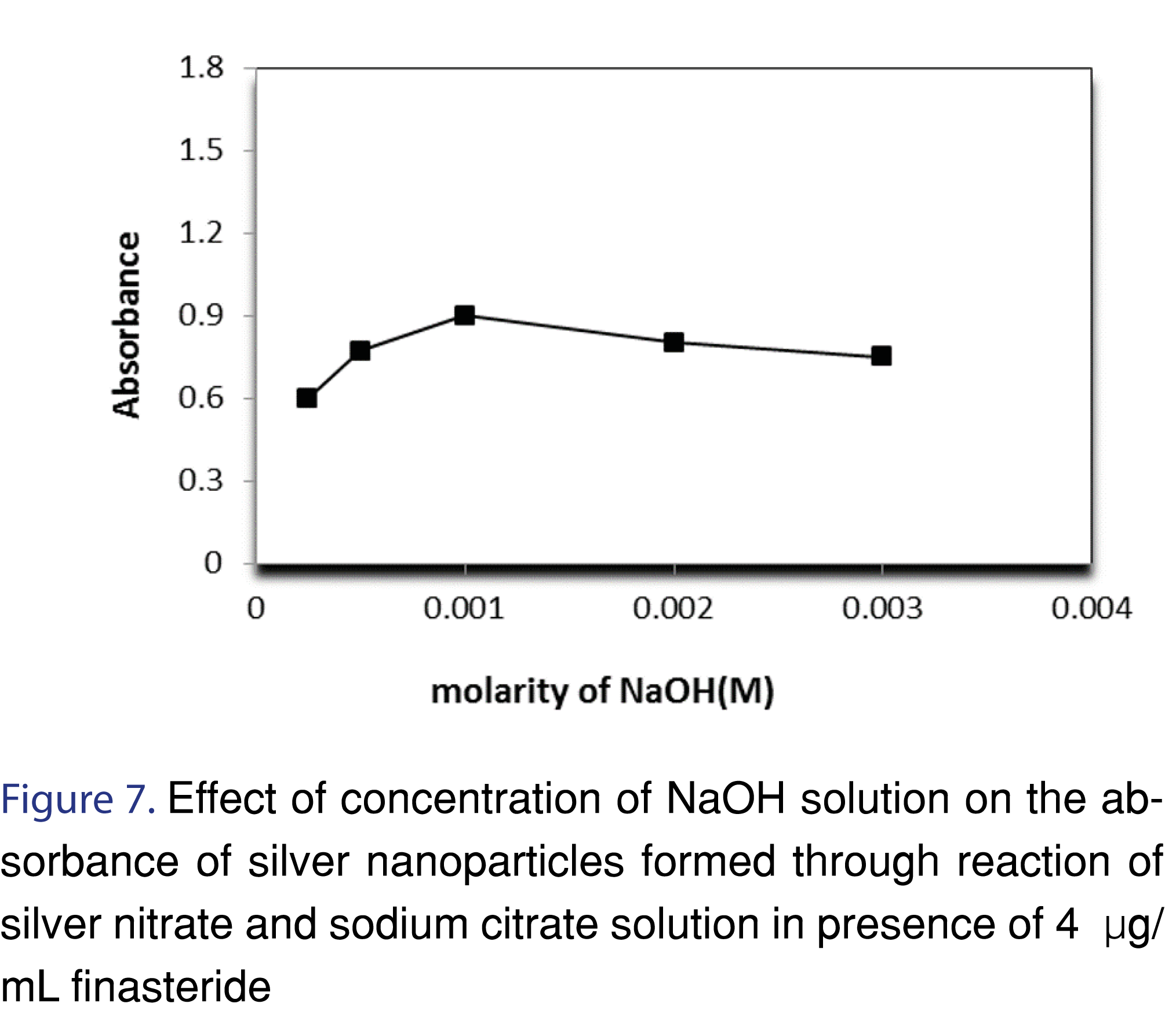
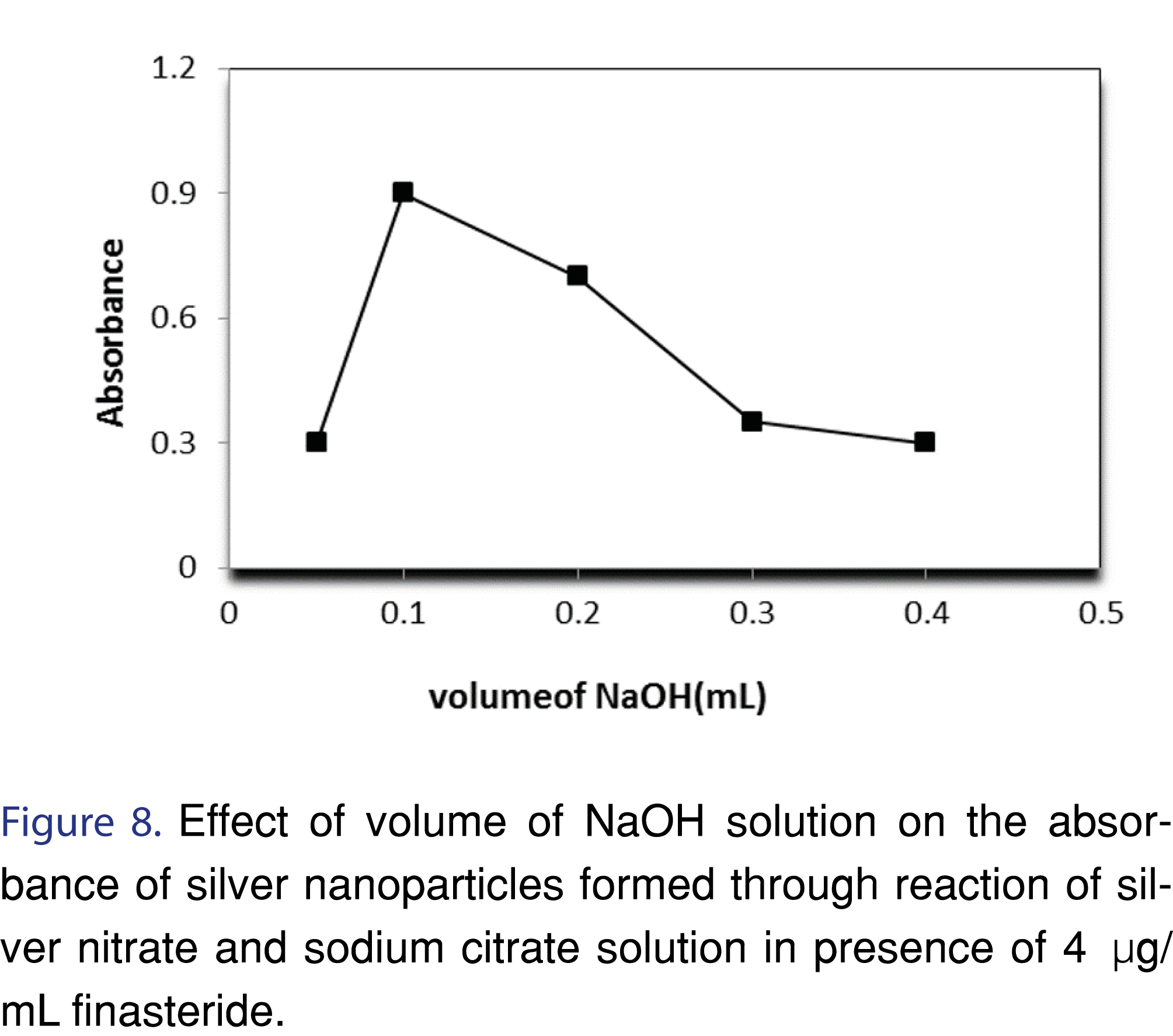
3.1.3 Effect of NaOH concentration
There was an increase observed in the absorbance by increasing NaOH concentration until a concentration of 0.001 M. Beyond this concentration, addition of NaOH showed decrease in the absorbance, and formation of black precipitates, most likely due to formation of silver oxide. The explanation of this observation can be as follow; the reaction between analyte and silver nitrate results in the formation of protons, as a result, the removal of these protons can enhance the formation of AgNPs (Figures 7,8 and Table 2).
3.1.4 Effect of temperature and time of heating
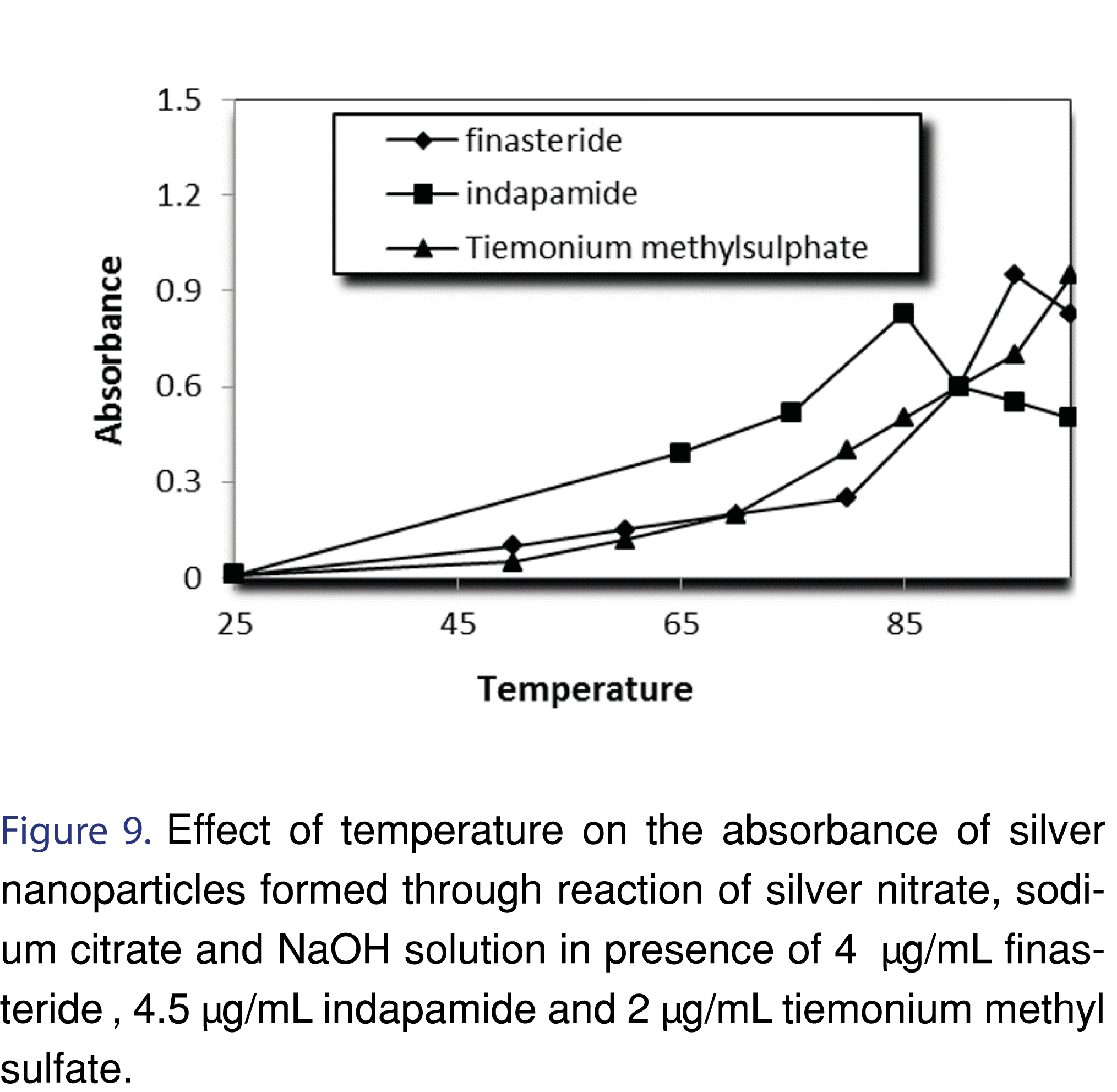
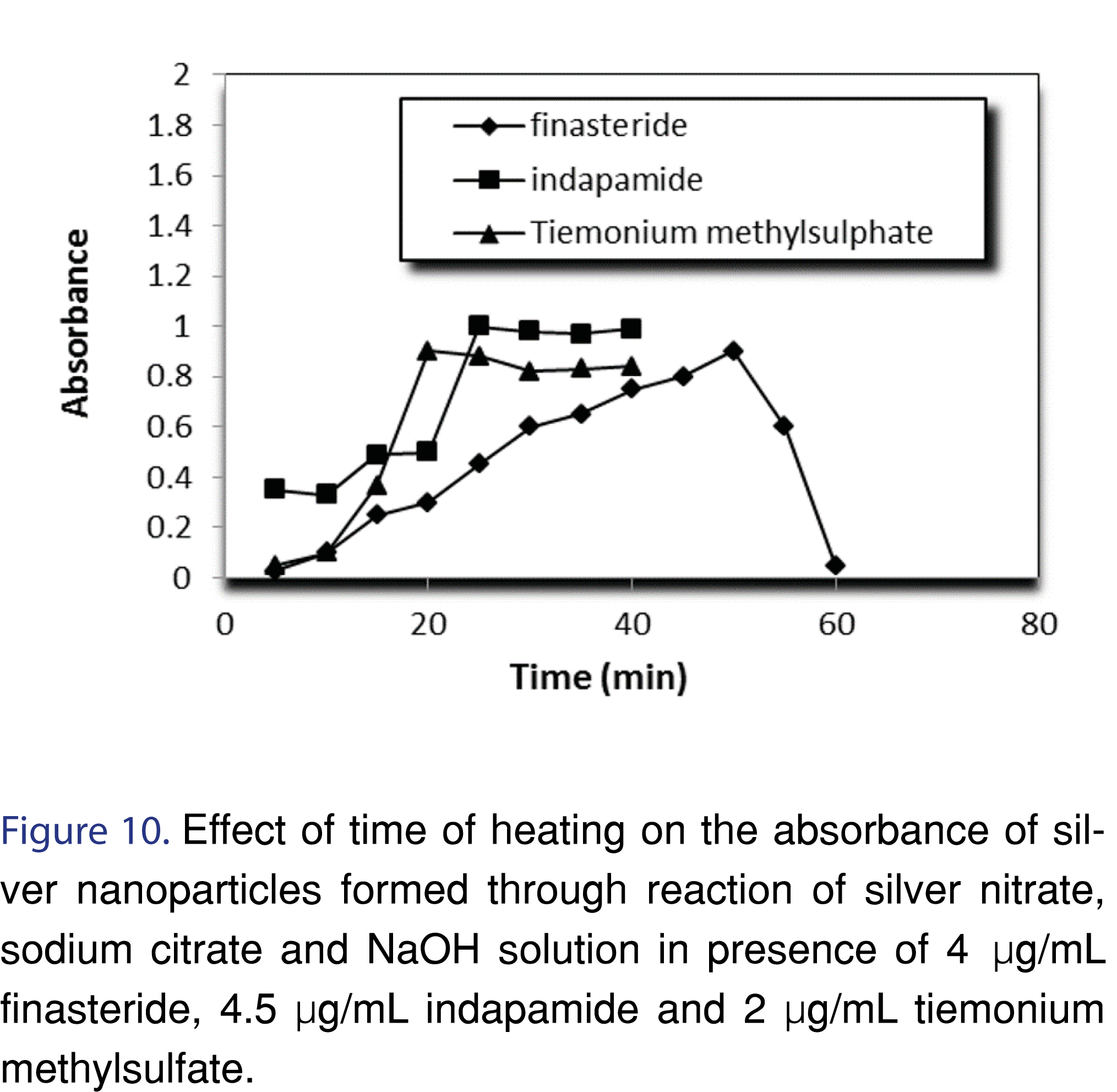
It was observed that the reaction rate increased with increasing temperature. Heating the solution in a water bath at 95°, 85° and 100°C for 50, 25 and 20 min was sufficient to produce maximum color intensities for finasteride, indapamide and tiemonium methylsulfate respectively. (Figures 9, 10 and Table 2).
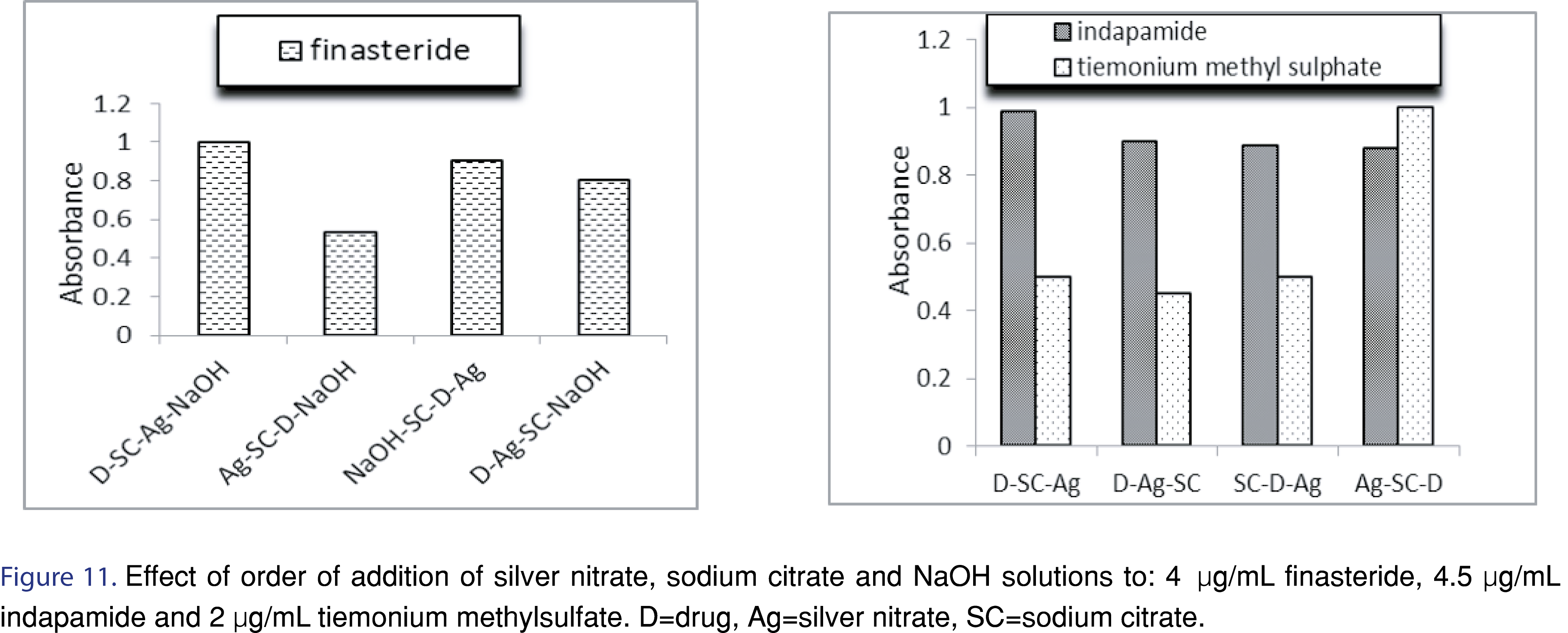
3.1.5 Order of addition
The sequence of the addition of reactants could influence the rate of silver nanoparticles formation. Out of several reagents studies, the most suitable sequence was drug, sodium citrate, silver nitrate then NaOH for finasteride, drug, sodium citrate then silver nitrate for Indapamide while silver nitrate, sodium citrate, drug for tiemonium methylsulfate (Figure 11).
It was observed that the reaction rate increased with increasing temperature. Heating the solution in a water bath at 95°, 85° and 100°C for 50, 25 and 20 min was sufficient to produce maximum color intensities for finasteride, indapamide and tiemonium methylsulfate respectively. (Figures 9, 10 and Table 2).
3.2. Method validation
Method validation was performed according to the ICH guidelines [35].
3.2.1 Linearity
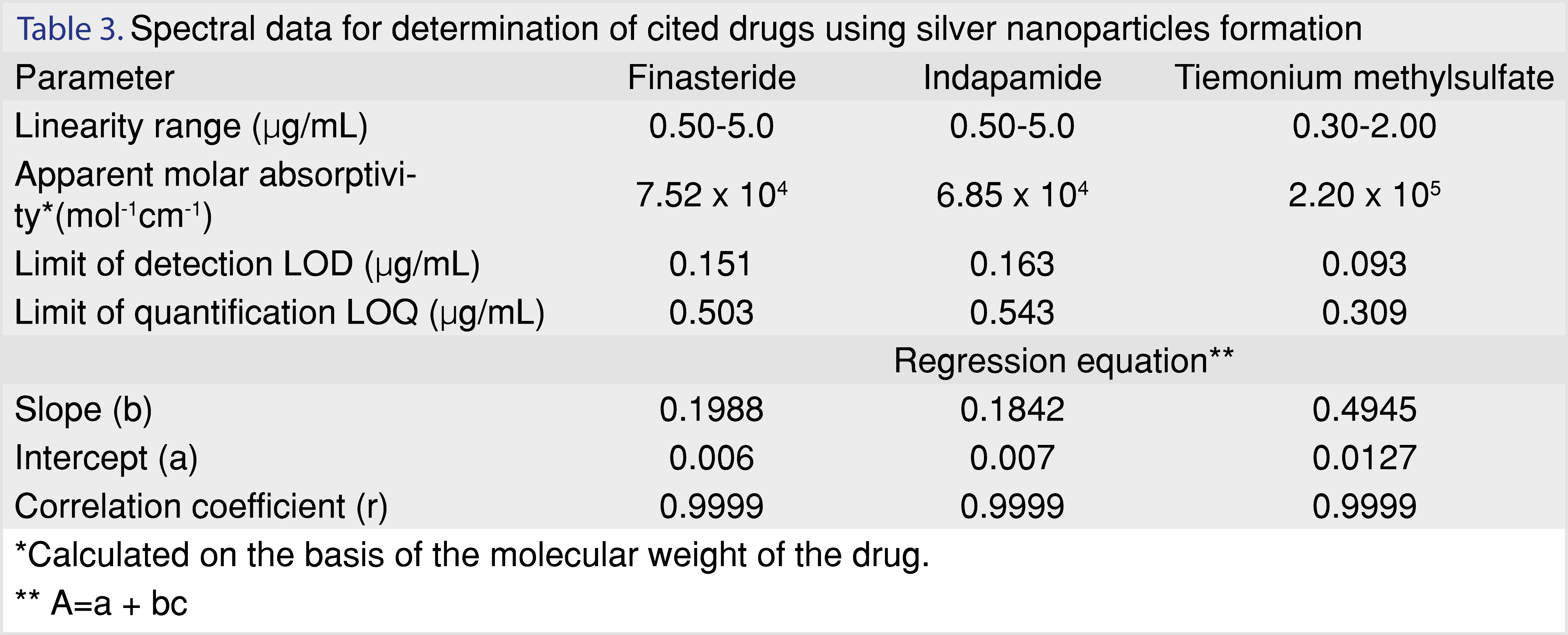
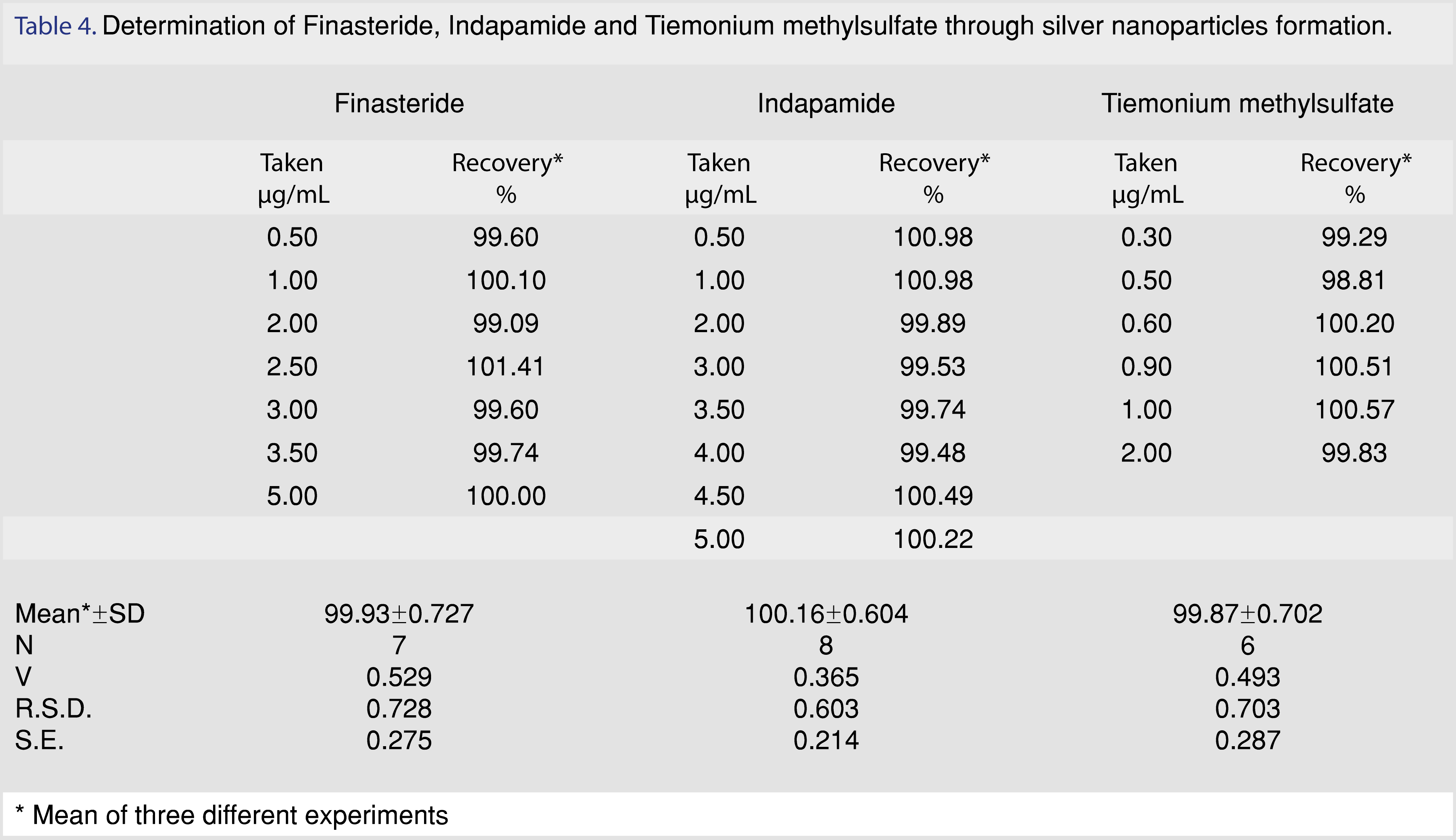
The linearity range of the cited drugs was (0.50-5.0 μg/mL), (0.50-5.0 μg/mL) and (0.30-2.0 μg/mL) for finasteride, indapamide and tiemonium methylsulfate respectively. Regression equation parameters were calculated. The small values of intercepts, relative standard deviation, standard error and high value of correlation coefficient indicated good linearity of the method. All these data were listed in Tables 3 and 4.
3.2.2 Sensitivity
The LOD and LOQ were calculated according to the following equation: LOD = 3.3(σ/s) and LOQ = 10(σ/s), where, σ = the standard deviation of blank and s = slope of the calibration curve. Their values were listed in Table 3 indicating the sensitivity of the proposed method.
3.2.3 Accuracy and precision
3.2.3.1 Accuracy
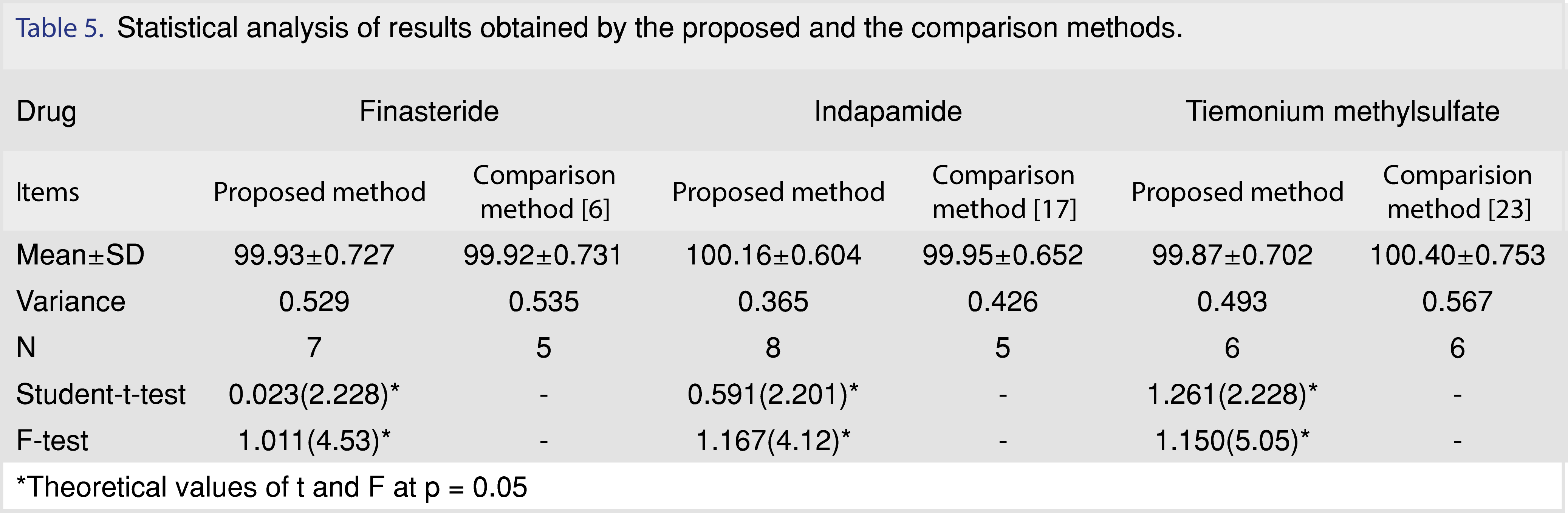
To ascertain the accuracy of the proposed method, the obtained results were compared with the reported methods. Statistical comparison of the results was performed using student t-test and F-test at 95% confidence level Table 5. No significant differences were found between the proposed methods and the reported ones.
3.2.3.2 Precision
Precision was determined by analyzing three different concentrations of each drug three successive times in the same day (intra-day). The same concentrations were assayed on three different days (inter-day). The relative standard deviation and percentages relative error (Er%) were calculated using the following equation:
Er% = [(found-added)/added] × 100%
Good results and acceptable relative standard deviations were obtained (Table 6).
3.2.4 Selectivity
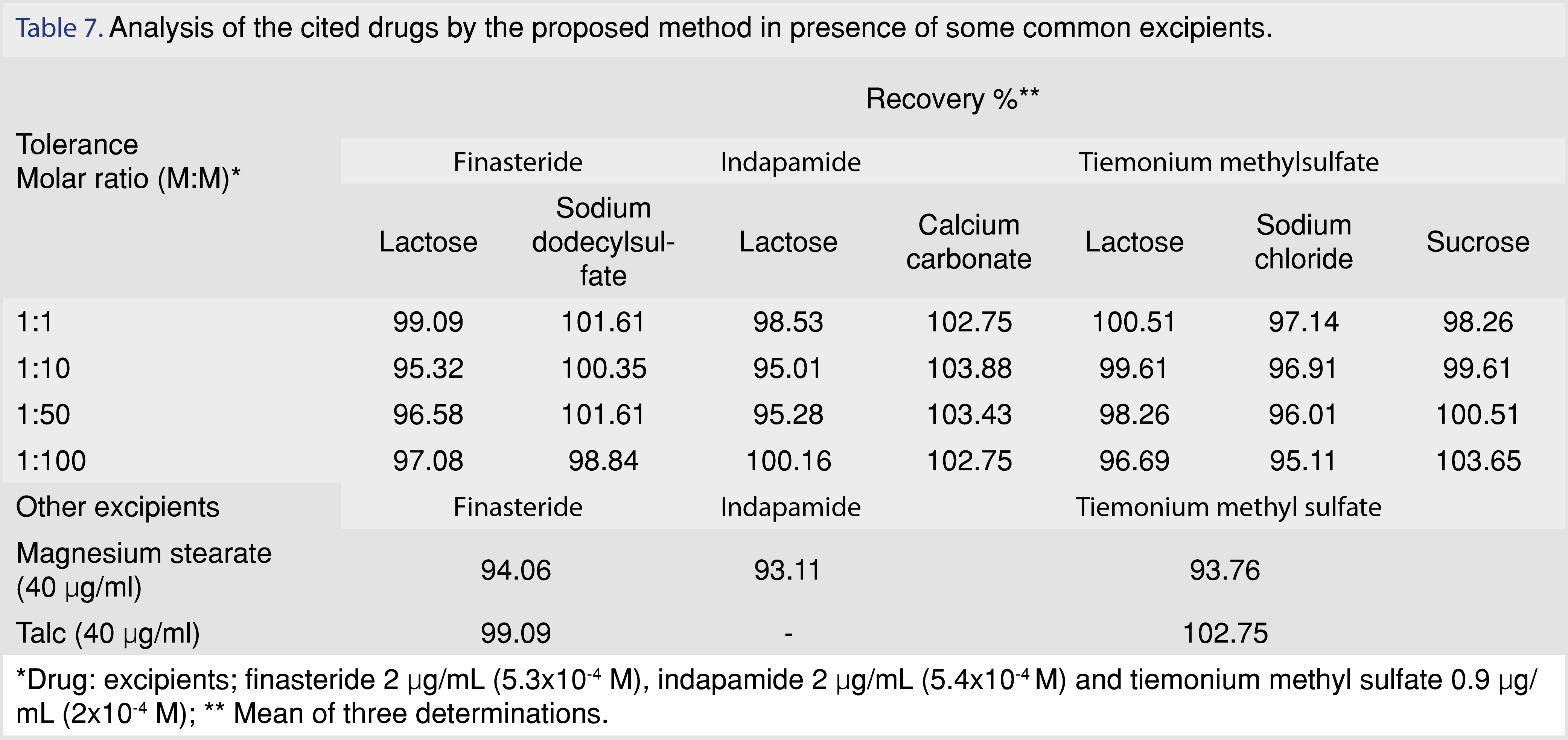
The selectivity of the method was checked by analyzing different mixtures of the cited drugs with some common excipients as lactose, sodium dodecyl sulphate, calcium carbonate, sodium chloride, sucrose, magnesium stearate and talc. Results showed some interferences from the presence of magnesium stearate which could be overcome by extraction with methanol for indapamide and tiemonium methylsulfate or ethanol for finasteride for tablets filtration Table 7.
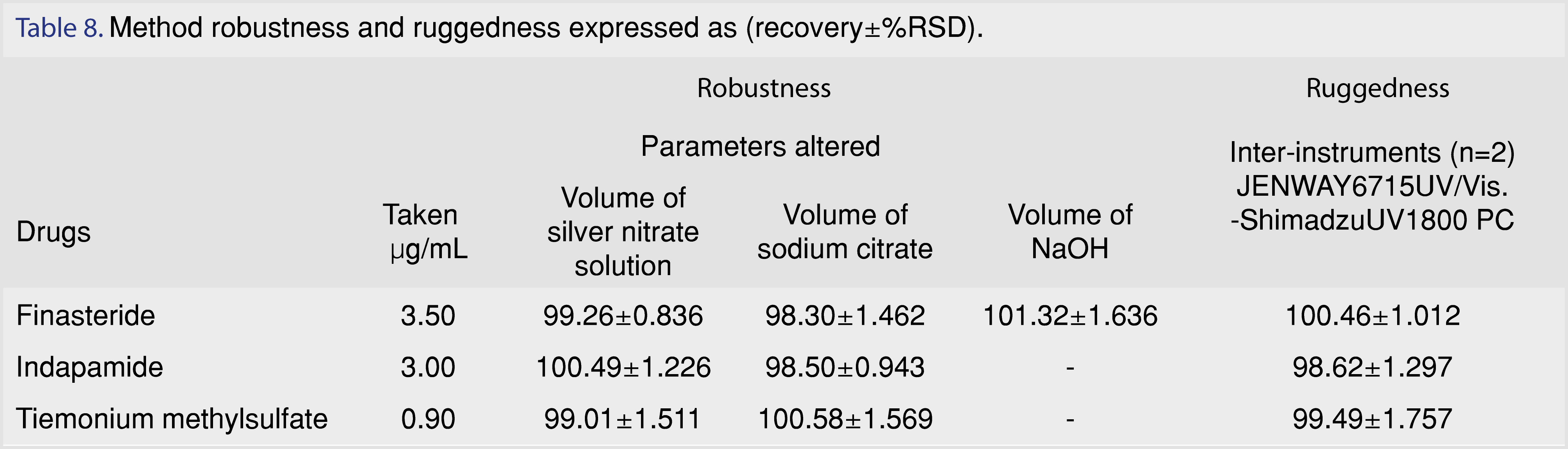
3.2.5 Robustness and ruggedness
Robustness was examined by evaluating the effect of small variations in the experimental parameters on the analytical performance of the proposed method. The variation of the studied parameters were analyzed according to the following: volume of silver nitrate solution (0.45, 0.40, 0.35 for finasteride, 1.1, 1.0, 0.9 mL for indapamide, 0.35, 0.30, 0.25 mL for tiemonium methylsulfate), sodium citrate volume (0.11, 0.10, 0.09 mL for Finasteride, 0.45, 0.40, 0.35 mL for Indapamide and 0.55, 0.50, 0.45 mL for tiemonium methyl sulfate) and NaOH volume (0.11, 0.10, 0.09 mL for Finasteride and 0.22, 0.20, 0.18 mL for tiemonium methylsulfate). It was found that these variations had a negligible influence on the results Table 8. Ruggedness was tested using two different instruments. The results were calculated as recovery ± %RSD. The low values of %RSD indicated the reproducibility of the method as shown in Table 8.
3.3 Analytical applications
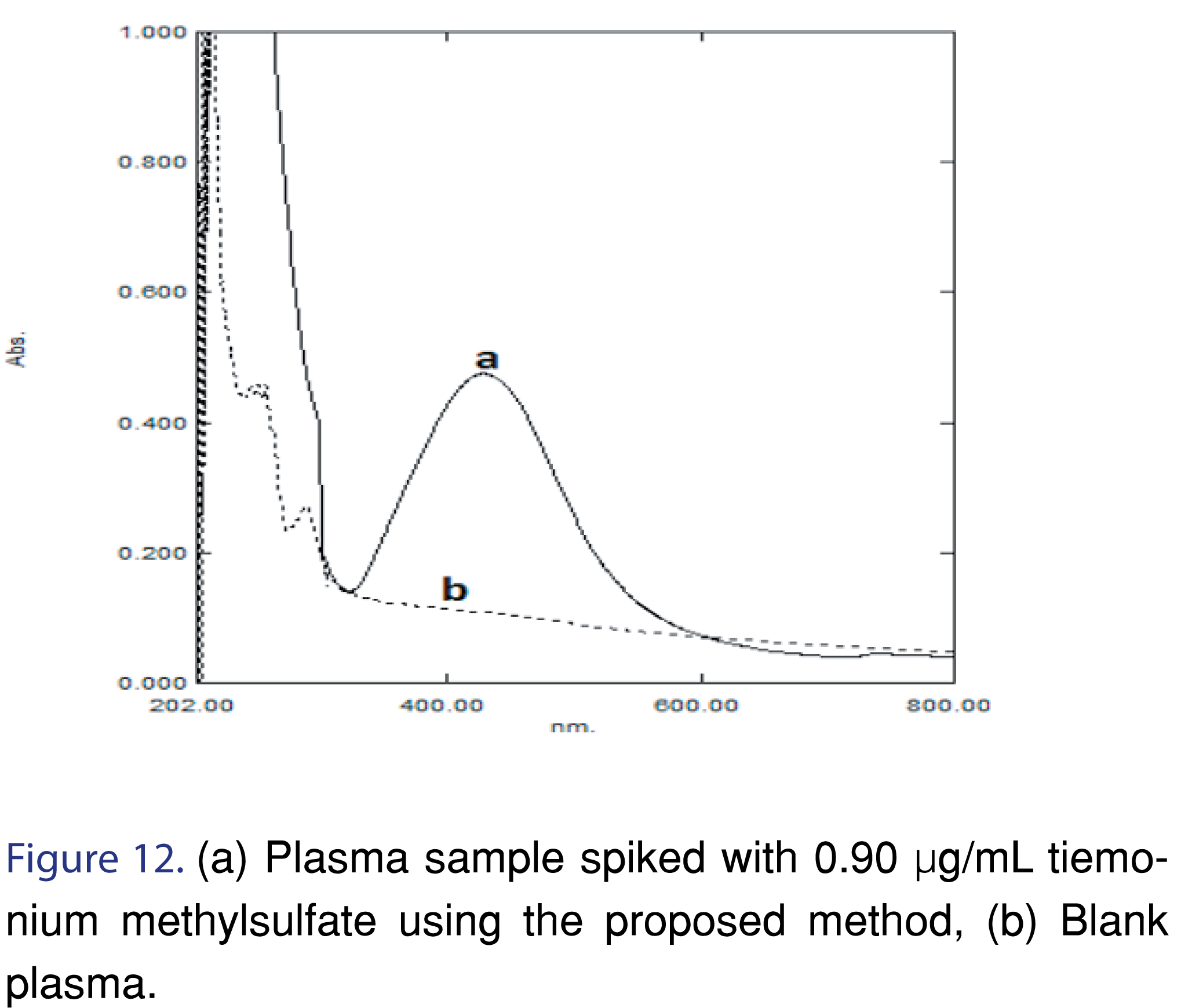
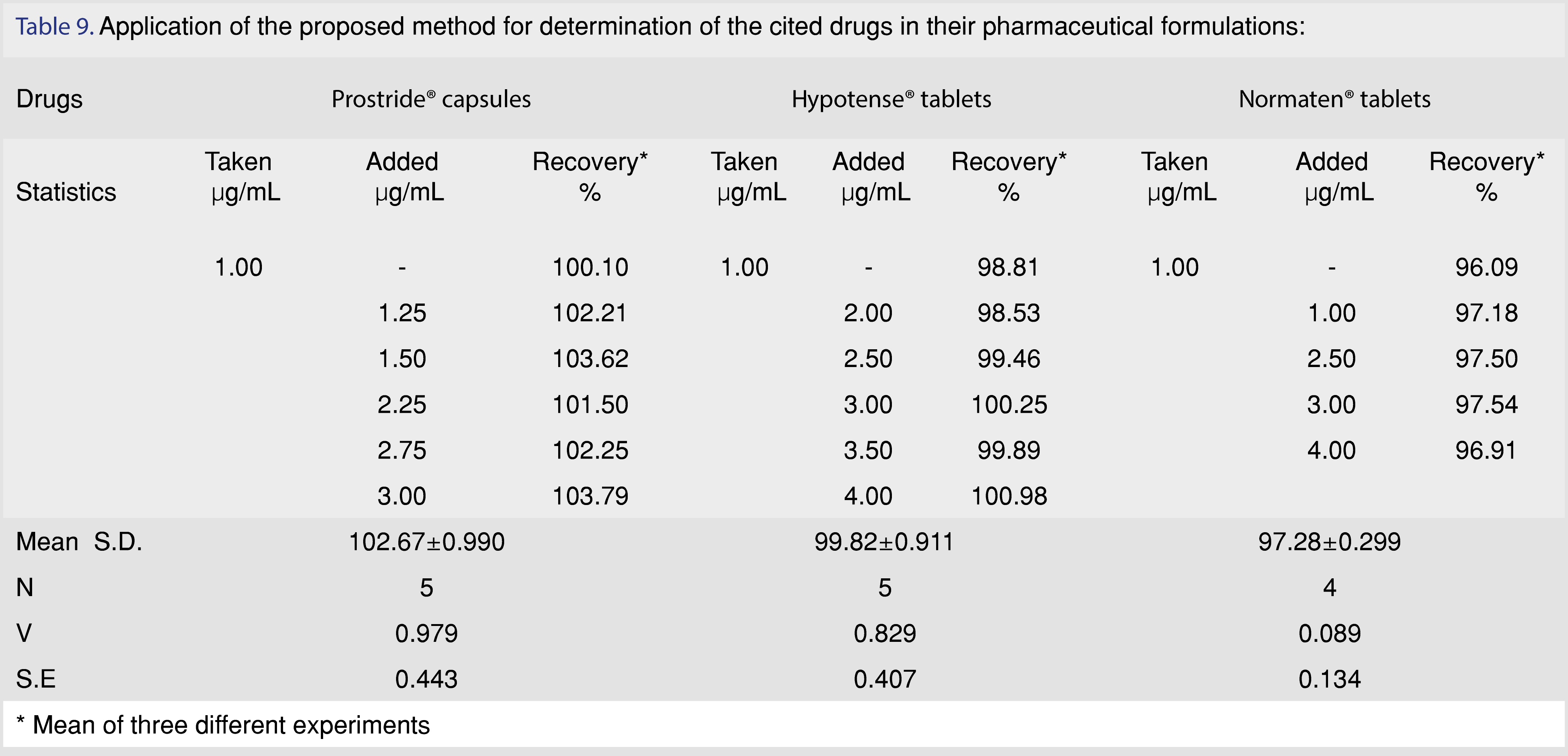
In the assay of Hypotense® and Normaten® tablets, first, we got a low recovery of indapamide but when we used 1.0 mL of 0.05 M HCl in dissolution medium, we got satisfactory results. This can be explained by a paper published by Nishath Fathima et al [36]. They studied mechanisms of drug excipient interaction showing that a physical interaction occurs between primary amine drugs, indapamide, and microcrystalline cellulose, an excipient in its tablet dosage forms. For low dose drugs, it can lead to dissolution failures and this can be remedied by carrying out dissolution using 0.05 M HCl). The proposed method was applied to determine the studied drugs in their pharmaceutical dosage forms with satisfactory results obtained. Also spiking of tiemonium methyl sulphate into plasma and good extraction from it proved the suitability of the proposed method Figure 12, Table 9-10.
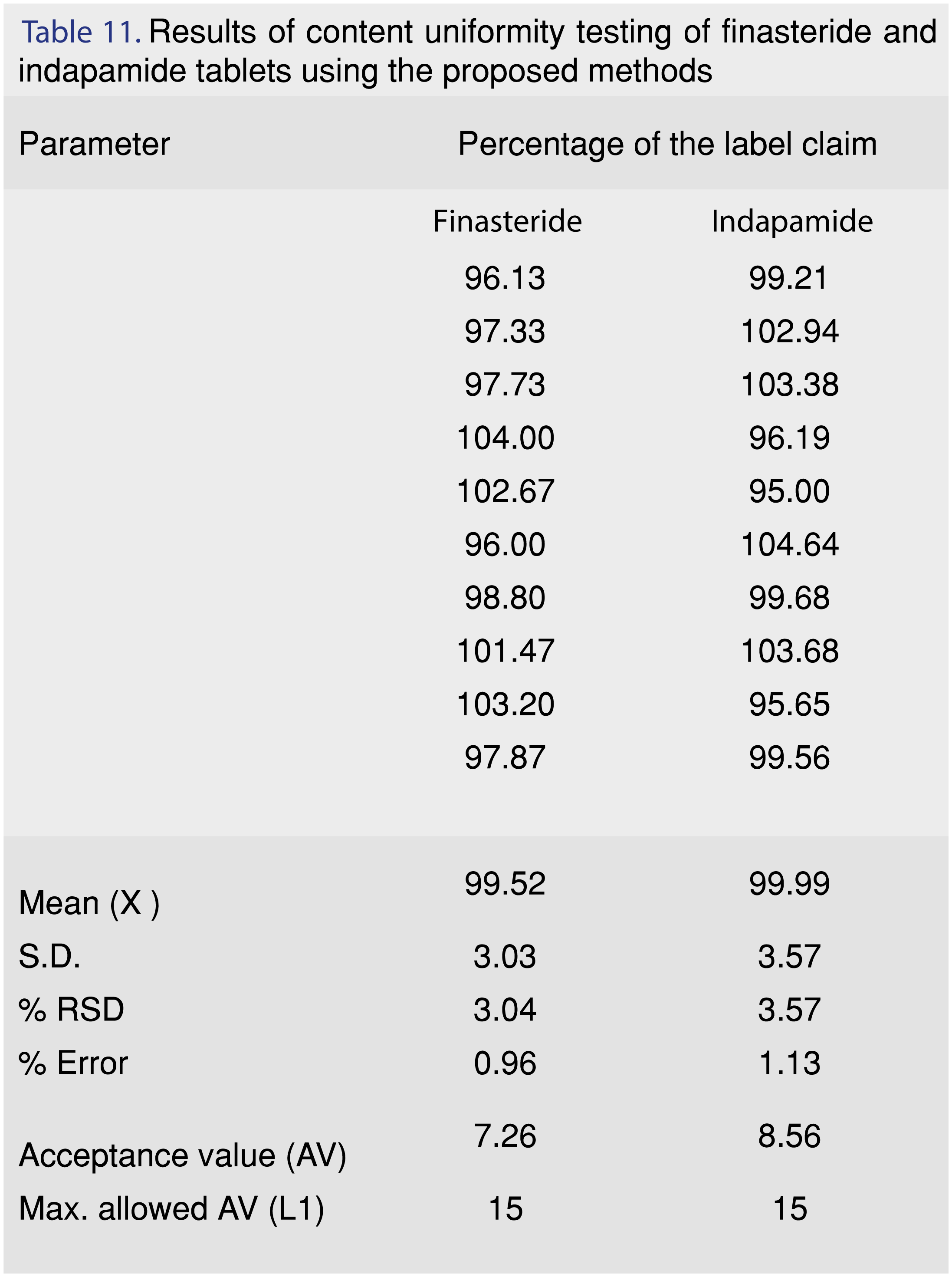
3.4 Content Uniformity Test
Due to the sensitivity of the proposed method, the method is ideally suited for content uniformity testing. The steps of the test were adopted according to the USP [37] procedure. The acceptance value (AV) was calculated and it was found to be smaller than the maximum allowed acceptance value (L1). The results demonstrated drug uniformity for finasteride and indapamide as shown in Table 11.
4.0 Conclusion
Application of silver nanoparticles as a chromogenic agent has been demonstrated in this work for optical determination of finasteride, indapamide and tiemonium methylsulfate. The proposed method was found to be simple, sensitive and easily applicable to the analysis of the cited drugs in their pharmaceutical dosage forms with good accuracy and precision. The method is based on the reduction of AgNO3 by the cited drugs.
5.0 Acknowledgments
6.0 References
- Sweetman SC. Martindale-the complete drug reference. 35 ed. London: The Pharmaceutical Press; 2188, 1314, 1776 (2007).
- The British Pharmacopoeia. Vol. II, III. Her Majesty’s Stationery Office: London, UK; (2009).
- Basavaiah K, Somashekar B C, Anilkumar U R, Ramakrishna V. Sensitive bromatometric assay methods for finasteride in pharmaceuticals. Ecletica Quimica.31(3), 31-38 (2006). [CrossRef]
- Kategaonkar AH , Patel DM, Choudhari VP, Kuchekar BS, Nikalje AG. Simultaneous determination of finasteride and tamsulosin in pharmaceutical preparations by ratio derivative spectroscopy, J Pharm Res 2(6), 1065-1067 (2009).
- Thimmaraju MK, Rao V, Gurrala S, Reddy GJ. UV spectrophotometric method for simultaneous determination of finasteride in bulk and pharmaceutical dosage form. Int J Pharm Bio Sci 1(3), 39-43 (2011).
- Vijaya LN, Rao GS, Rani BR, Manasa K, Prasad VB. Development and validation of UV spectrophotometric method for the estimation of finasteride in tablets. Int J Pharma Sci 3(1), 123-125 (2013).
- Ahmed SM, Elbashir AA. Development and validation of spectrophotometric method for determination of finasteride in pharmaceutical formulation using 1, 2-naphthoquine-4-sulfonate (NQS). J Anal Bioanal Tech. 6(3), 1-5 (2015). [CrossRef]
- Segall AI, Vitale MR, Perez VL, Palacios ML, Pizzorno MT. A stability-indicating HPLC method to determine finasteride in a tablet formulation. J Liquid Chromatogr Relat Technol 25(20), 3167-3176 (2002). [CrossRef]
- Demir H, Cucu A, Sakarya S. Determination of finasteride in the tablet form by liquid chromatography and its analytical method validation. Anal Chim Acta 557(1-2), 252-255 (2006). [CrossRef]
- Sindhura M, Raghavi K, Prashanth I, Buchi NN. Rapid analysis of finasteride in bulk and formulations by RP-HPLC-PDA method. J Chil Chem Soc 57 (4), 1469-1471 (2012). [CrossRef]
- Bari S B, Jain P S, Bakshi A R, Surana S J. HPTLC method validation for simultaneous determination of tamsulosin hydrochloride and finasteride in bulk and pharmaceutical dosage form. J Anal Bioanal Tech 2(2), 2-5 (2011).
- Alvarez-Lueje A, Brain-Isasi S, Núñez-Vergara LJ, Squella JA. Voltammetric reduction of finasteride at mercury electrode and its determination in tablets. Talanta 75(3), 691-696 (2008). [CrossRef]
- Amer SM. Polarographic behavior and determination of finasteride. Farmaco 58(2) 159-163 (2003). [CrossRef]
- Agrawal Y K, Majumdar FD. Spectrophotometric determination of indapamide and its formulations using ammonium molybdate reagent. Anal Lett 28(2), 1619-1627 (1995). [CrossRef]
- Youssef NF. Spectrophotometric, spectrofluorimetric, and densitometric methods for the determination of indapamide. J. AOAC Int 86(5), 935-940 (2003).
- Pai J B, Shetty SK, Henna GP, Gopinath B, Ahmed M. Development of new spectrophotometric methods for the determination of indapamide in bulk and pharmaceutical formulations. Int J Chem Tech Res 3(2), 755-760 (2011).
- Tarkase KN, Jadhav MB, Tajane SR, Dongare US. Development and validation of UV-spectrophotometric methods for estimation of indapamide in bulk and tablet dosage form. Der Pharma Chemica 4(3), 1128-1132 (2012).
- odeschini V, Leite R, Sangoi M, Oliverira P, Barth T. Multicomponent spectrophotometric method for simultaneous analysis of delapril and indapamide in tablets. Drug Anal Res 1(1), 50-55 (2017). [CrossRef]
- Hang T, Zhang Y. A selective HPLC method for the determination of indapamide in human whole blood: applicaton to a bioequivalence study in chinese volunterrs. J Pharm Biomed Anal 40(1) 202-205 (2006). [CrossRef]
- Moussa BA, Abadi AH, Abou Youssef H, Mahrouse MA. Development and validation of a stability indicatimg HPLC method for the simultaneous determination of captopril and indapamide. Anal Chem Ind J 12(6), 222-232 (2013).
- El-Bagary R, Elkady EF, Mowaka S, Attalah MA. A validated HPLC method for simultaneous determination of perindopril, arginine, amlodipine and indapamide application in bulk and in different pharmaceutical dosage forms. J AOAC Int 100(4), 992-999 (2017). [CrossRef]
- Radi A. Stripping voltammetric determination of indapamide in serum at castor oil-based carbon paste electrode. J Pharm Biomed Anal 24(3), 413-419 (2001). [CrossRef]
- Saiful Islam M, Wahiduzzaman, M, Shafiqul Islam M, Rafiquzzaman, Kundu S K. UV spectroscopic method for estimation of tiemonium methyl sulfate 50 mg tablet in bulk and pharmaceutical preparations. Int Pharm Sci Res 5(2), 548-555 (2014).
- Zaazaaa HE, Abbasa SS, EL-Sheriff ZA, El-Zeanya B,
EL Haddadb DA. Stability indicating spectrophoto
metric methods for determination of tiemonium methyl sulphate in the presence of its degradation products. J Appl Pharm Sci 4(1), 033-045 (2014). [CrossRef] - Ramadan NK, Abd El Halim LM, EL Sanabary HFA, Salem MY. Stability indicating spectrophotometric methods for the determination of tiemonium methyl sulphate. Int J Drug Develop Res 6(1),160-168 (2014).
- Ayad MM, El-Balkiny MN, Hosny MM, Metias YM, Spectrophotometric determination of tiemonium methyl sulfate, itopride hydrochloride and trimebutine maleate via ion pair complex formation and oxidation reaction. Ind J Adv Chem Sci 4(1), 85-97 (2016).
- Ayad MM, A Abdellatef HE, Hosny MM, Kabil NS, Spectrophotometric determination of etilefrine HCl, salbutamol sulphate and tiemonium methyl sulphate using surface plasmon resonance band of gold nanoparticles. Nano Biomed Eng 10(1), 16-24 (2018). [CrossRef]
- Nesrin KR, Lamia MA, Hoda FA, Maissa YS. Stability indicating chromatographic methods for the determination of tiemonium methyl sulphate. Int J Adv Res 2(1), 366-376 (2014).
- Ayad MM, El-Balkiny MN, Hosny MM, Metias YM, Green validated method for determination of tiemonium methyl sulfate using reversed-phase high-performance liquid chromatography technique with stability-indicating studies, Inn J Med Sci 4(3), 1-9 (2016).
- Ayad MM, El-Balkiny MN, Hosny MM, Metias YM, Conductometric determination of tiemonium methylsulfate, alizapride hydrochloride, trimebutine maleate using rose bengal, ammonium reineckate and phosphotungstic acid. Ind J Adv Chem Sci 4(2), 149-159 (2016).
- Rahman MM, Khan SB, Asiri AM, Alamry KA, Al-Youbi AO. Detection of nebivolol drug based on as-grown un-doped silver oxide nanoparticles prepared by a wet-chemical method. Int J Electrochem Sci 8,323-335 (2013).
- Rahnama MR. Determination of fexofenadine using silver nanoparticles by spectrophotometric method. Int J Chem Tech Res 5, 2508-2512 (2013).
- Tashkhourian J, Nezhad MRH, Khodaveisi J. Application of silver nanoparticles and principal component-artificial neural network models for simultaneous determination of levodopa and benserazide hydrochloride by a kinetic spectrophotometric method. Spectrochim Acta Part A. 82, 25-30 (2011). [CrossRef]
- Ayad MM, Abdellatef HE, Hosny MM, Sharaf YA. determination of etilefrine hydrochloride, fenoterol hydrobromide, salbutamol sulphate and estradiol valerate using surface plasmon resonance band of silver nanoparticles. Int J Pharmacy Pharm Sci 7(5), 327-333 (2015).
- ICH. Q2A (R1). Validation of Analytical Procedures: Text and Methodology. International Conference on Harmonisation. Geneva, Switzerland, (2005).
- Fathima N, Mamatha T, Qureshi HK, Anitha N, Rao JV. drug-excipient interaction and its importance in dosage form development. J Appl Pharm Sci 01 (06),66-71(2011).
- The United States Pharmacopeia, 3rd ed., the United States Pharmacopoeial Convention, Rockville, MD, USA (2011).
All site content, except where otherwise noted, is licensed under a Creative Commons Attribution 4.0 License